Abstract
New Delhi metallo-beta-lactamase (NDM-1) is an enzyme that makes bacteria resistant to a broad range of beta-lactam antibiotic drugs. This is because it can inactivate most beta-lactam antibiotic drugs by hydrolyzing them. For in-depth understanding of the hydrolysis mechanism, the three-dimensional structure of NDM-1 was developed. With such a structural frame, two enzyme-ligand complexes were derived by respectively docking Imipenem and Meropenem (two typical beta-lactam antibiotic drugs) to the NDM-1 receptor. It was revealed from the NDM-1/Imipenem complex that the antibiotic drug was hydrolyzed while sitting in a binding pocket of NDM-1 formed by nine residues. And for the case of NDM-1/Meropenem complex, the antibiotic drug was hydrolyzed in a binding pocket formed by twelve residues. All these constituent residues of the two binding pockets were explicitly defined and graphically labeled. It is anticipated that the findings reported here may provide useful insights for developing new antibiotic drugs to overcome the resistance problem.
Introduction
The rapid growth of antibiotic resistance has become a main clinical and epidemiological problem for human health [1]. In bacteria, β-lactam antibiotics are primarily hydrolyzed by β-lactamases in an acylation-deacylation-based process [2]. Thus, it is believed that β-lactamases play an important role in leading to resistance of bacteria to β-lactam antibiotics. These enzymes are capable of cleaving the amide bond of the β-lactam ring so as to inactivate the β-lactam antibiotic drugs. According to their sequence similarities, β-lactamases can be generally divided into four classes, named as A, B, C, and D. Classes A, C, and D of β-lactamases contain serine groups in their active sites, while the enzymes in class B are metalloproteins, or called “metallo-β-lactamases”, that require one or two zinc ions for their activity. Among all the β-lactamases, metallo- β-lactamases are the major culprit causing bacteria to resist antibiotics, due to the reason that they can degrade all β-lactams except monobactams and that they are special for their constant and efficient carbapenemase activity [3].
In December 2009 a novel metallo-β-lactamase was identified in a patient hospitalized in New Delhi with an infection caused by klebsiella pneumonia [4]. This β-lactamase was later detected in bacteria in India, Pakistan, United Kingdom, United States, and Canada. The New Delhi metallo-β-lactamase (NDM-1) has the ability to make bacteria resistant to a wide range of β-lactam antibiotics, including the carbapenem family antibiotics that are a mainstay for the treatment of antibiotic-resistant bacterial infections [5], [6]. According to the report of the United Kingdom's Health Protection Agency, most isolates with NDM-1 are resistant to all standard intravenous antibiotics for the treatment of severe infections. The most common bacteria that make this enzyme are Gram negative such as Escherichia coli and Klebsiella pneumonia, but the gene for NDM-1 can spread from one strain of bacteria to another by horizontal gene transfer.
To reveal the resistance mechanism of bacteria to β-lactam antibiotics due to the existence of NDM-1, an indispensable knowledge is of the 3D (three-dimensional) structure of NDM-1. Since so far no 3D structure whatsoever has been determined by experiments for NDM-1, we have to resort to the approach of structural bioinformatics [7]. Recently, growing evidences have indicated that various tools in structural bioinformatics, such as homology modeling [8], [9], [10], [11], [12], [13], [14], [15], [16], molecular docking [17], [18], [19], [20], [21], [22], as well as molecular dynamics simulations [23], [24], [25], [26], [27], [28], [29], [30], [31], can timely provide very useful information and insights for biomedical science and drug development and hence are quite rewarding [14], [22], [29], [32], [33], [34], [35], [36], [37], [38], [39]. In view of this, the present study was initiated in an attempt to develop a homology model for NDM-1, based on which the molecular docking operations and molecular dynamics simulations were performed in hopes that the information thus obtained may provide useful insights or clues for designing new drugs to overcome the antibiotic resistance problem.
Materials and Methods
The entire sequence of NDM-1, which contains 158 amino acids, was taken from NCBI Protein database with an accession of AB571289. According to the score of BLAST search, the crystal structure of VIM-2, a Zn-β-lactamase from Pseudomonas aeruginosa [40], was selected as a structural template to perform homology modeling to develop the 3D structure of NDM-1. The PDB code of the crystal structure is 1ko3, which was released in 2008 with a resolution of 2.20 Å [40]. The entire sequence of 1ko3 contains 230 amino acids. The sequence alignment between NDM-1 and 1ko3 was performed by the Molecular Operating Environment (MOE), and the alignment result thus obtained indicates that the two proteins have a sequence identity of 43%.
Meanwhile, using the web-server EzyPred [41] at http://www.csbio.sjtu.edu.cn/bioinf/EzyPred/ and the protein sequence information, it was identified that NDM-1 is a member of hydrolases enzyme family (acting on carbon-nitrogen bonds other than peptide bonds), and so is 1ko3. Since both NDM-1 and 1ko3 belong to a same enzyme family with the same action mechanism, and their sequence identity is higher than 40%, it is quite reasonable to use the crystal structure of 1ko3 [40] as a template to develop the 3D structure of NDM-1 via homology modeling.
Based on the sequence alignment (Fig. 1) as well as the atomic coordinates of 1ko3, the 3D structure of NDM-1 was derived by using the I-TASSER algorithm, an extension of the previous TASSER (Threading/Assembly/Refinement) method [42], [43], [44]. The homolog-modeled 3D structure was subject to a short-time molecular dynamics simulation (∼3 ns) for further refinement. The final 3D structure thus obtained for NDM-1 is shown in Fig. 2. Meanwhile, some assessments by the Swiss Model Server indicated that the computed structure is quite reasonable and creditable.
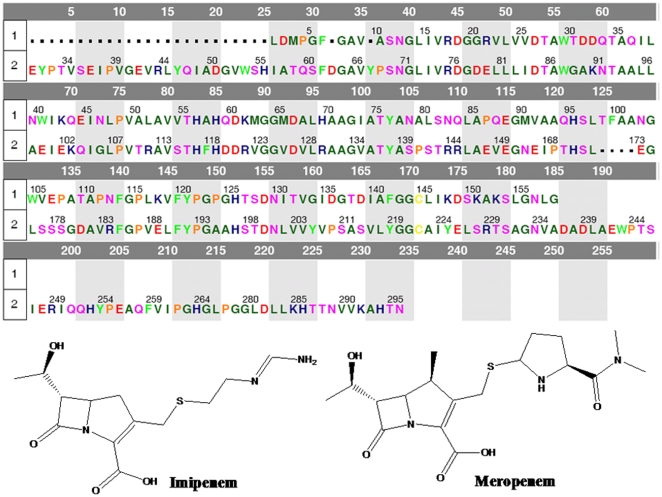
Figure 1. The sequence alignment of NDM-1 (chain-1) with 1ko3.pdb (chain-2); shown in the lower panel are the chemical structures of Imipenem and Meropenem.
The amino acids in this figure are colored according to the following four types: (1) acidic, red; (2) basic, dark blue; (3) hydrophobic, green; (4) hydrophilic, light blue. The sequence identity of the two proteins is about 43%.
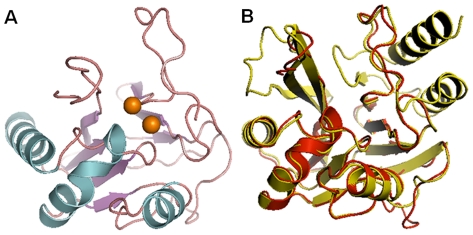
Figure 2. Illustration to show (A) the computed 3D structure of NDM-1 that contains three helices and 7 β-strands, and (B) a superimposition of the homology modeled NDM-1 structure (red) with its template 1ko3 (yellow).
Subsequently, molecular docking operations were carried out with Monte Carlo simulated annealing [45] to get the favorable binding interaction modes for NDM-1 respectively with Imipenem and Meropenem, two typical β-lactam antibiotic drugs. It was reported recently [6] that NDM-1 showed a comparatively high resistance to Imipenem and Meropenem. The binding pocket was identified using Q-SiteFinder [46]. Because binding of inhibitors in the active site may induce a conformational change to closing some flaps [47], we adopted a flexible docking procedure to construct the binding modes of NDM-1 with Imipenem and Meropenem. Before the docking procedure, we extracted 1000 conformations of NDM-1 from the aforementioned 3-ns molecular dynamics simulation. The ligand (Imipenem or Meropenem) was then docked to all of these conformations to search for a favorable binding mode. The docking program [48] used in this study would automatically generate a diversified set of configurations by randomly changing the ligand's coordinates. When a new configuration of the ligand was generated, the search for the favorable binding was operated within a specified 3D box by the simulated annealing to optimize the purely spatial contacts as well as electrostatic interactions. Finally, the favorable binding mode thus obtained was further optimized by a short time molecular dynamics simulation (∼5 ns). The binding energy was calculated using a scoring function London dG [49], [50],[51]. In all our calculations, the Merck force field (FFMM94) parameters were adopted. The most favorable binding interactions thus obtained for NDM-1 with Imipenem and Meropenem are given in Fig. 3 and Fig. 4, respectively.
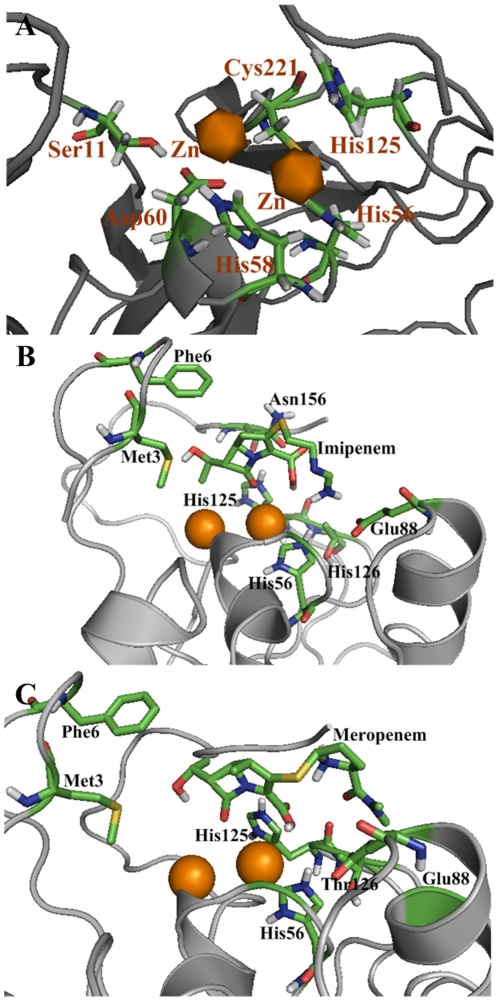
Figure 3. A close view to show (A) the zinc coordinates, (B) binding pocket of NDM-1 for the antibiotic drug Imipenem, and (C) binding pocket of NDM-1 for the antibiotic drug Meropenem.
The binding pocket is formed by those residues that have at least one heavy atom with a distance within 5 Å [51] to Imipenem/Meropenem.
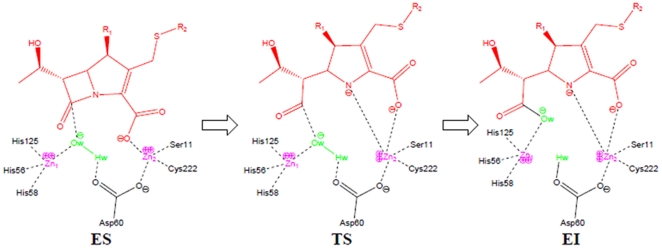
Figure 4. The proposed mechanism for the initial ring-opening step in the antibiotic catalysis of NDM-1, where ES, TS, and EI represent enzyme-substrate, transition state, and enzyme intermediate, respectively.
Results and Discussion
Homology-Modeled 3D Structure of NDM-1
A total of 100 homology-modeled structures for NDM-1 were derived with each having a C-score. The C-score is a “confidence score” for estimating the quality of a computed model: a high C-score signifies a model with a high confidence and vice-versa. Shown in Fig. 2A is the structure with the highest C-score that was used as a receptor for further docking studies. Meanwhile, we employed PROCHECK [52] to estimate the quality of our models. It was indicated by PROCHECK that there are 93.1% residues located in the “core” and “allowed” regions, 4.6% in the “general” region and only 2.3% in the “disallowed” regions (Fig. S1). In the computational structure, all the bond lengths for the main-chain residues and 91.9% bond angles for the main-chain residues are within the allowed limits. To further examine our computational model of NDM-1, we also used the tool of QMEAN, which is a scoring function of a linear combination of 6 structural descriptors [53]. The QMEAN score ranges between 0 and 1 with higher value to reflect a better quality of the model. The QMEAN score for the current computational model is 0.67, and the density plot of this QMEAN score is shown in Fig. S2. To evaluate the absolute model quality, we also calculated a Z-score of the computational model in comparison with the scores of the reference X-ray structures of similar size from the Protein Data Bank (Fig. S3). For each of the QMEAN components, a Z-score was computed in comparison with the average X-ray structures (the average value is 0, as shown in Fig. S4). Based on these analyses, we further examined the per-residue error (Fig. S5), visualized using a color gradient from blue (reliable regions with estimated error below 1 Å) to red (potentially unreliable regions with estimated error above 3.5 Å). We found that the residues in the potentially unreliable regions were mainly located on the loop regions in the computational model, indicating that further molecular dynamics optimizations are needed, as described below.
However, after a 3-ns molecular dynamics simulation, all the residues in our model were in the “core” and “allowed” regions. It can be seen from Fig. 2A that, like most of the other metallo-β-lactamases, the NDM-1 protein belongs to the α/β structural class [54], with 3 helices and 7 β-strands. The 3 helices were exposed to the solvent. The N-terminal and C-terminal regions of NDM-1 can be superposed by a 180 degrees rotation around a central axis, indicating that the entire structure may have arisen from the duplication of a gene. It was found after superposing NDM-1 to its template structure 1ko3.pdb that the two structures share a quite similar backbone (Fig. 2B) owe to their high sequence identity percentage. However, some α-helices and β-strands in 1ko3.pdb are not seen in the NDM-1 model because 1ko3.pdb contains 5 helices and 11 β-strands. This is due to the sequence deletion of NDM-1 in its C-terminal and N-terminal regions. It is instructive to note that such sequence deletion might make the β-lactam antibiotic drugs easier to access the binding pocket of NDM-1.
Similar with other metallo-β-lactamases, the active site of NDM-1 presents two metal ion binding sites: His site and Cys site. Thus, two zinc ions can be detected in our homology model at both the His and Cys sites with a distance of 4.20 Å apart. According to some experimental data, the one located in the His site has a higher occupancy (1.3 fold) than that in the Cys site (Fig. 3A). The zinc ion in the His site possesses a tetrahedral coordination sphere and is coordinated by His56, His58, and His125. The other zinc ion in the Cys site has a trigonal-pyramidal coordination sphere that involves Ser11, Asp60, and Cys144.
Binding Modes of Inactivating Antibiotic Drugs
Since metallo-β-lactamases can inactivate the β-lactam antibiotic drugs by cleaving the amide (carbon-nitrogen) bond of the β-lactam ring [2], it can provide us very useful insights about the essence of the drug resistance problem by analyzing the binding interaction modes obtained by docking Imipenem and Meropenem to NDM-1 receptor, respectively.
Shown in Fig. 3B is a close view of the binding interactions obtained by docking the antibiotic drug Imipenem to the receptor NDM-1. Its binding pocket is formed by nine residues that have at least one heavy atom with a distance within 5 Å [55] to Imipenem, as labeled in Fig. 3B. Interestingly, the constituent residues thus defined for the binding pocket are quite consistent those identified by the online web-server tool Q-SiteFinder for forming the extended cleft active site of NDM-1. In the NDM-1/Imipenem complex, Met3 and Phe6 are located on the brink of the active site, providing some van der Waals interactions to the drug molecule. It can be seen from Fig. 3B that, in addition to the van der Waals interaction of the Imipenem drug with the surrounding binding pocket residues, there are remarkable hydrogen bonds that have tightly tethered the drug to Glu88 and Thr126 of the receptor, making NDM-1 able to recognize the antibiotic drug followed by cleaving the amide bond of its β-lactam ring so as to inactivate Imipenem. The detailed information for these hydrogen bonds is listed in Table 1.
Table 1. Detailed information for the H bonding formed by NDM-1 and antibiotic drugs.
| H – donor | H – receptor | Bond distance (Å) | Lifetime (%) |
| Glu88 (Oβ) | Imipenem (NH2) | 2.89 | 43.8 |
| Glu88 (Oβ) | Meropenem (NH2) | 3.01 | 39.1 |
| Thr126 (Oα) | Imipenem (NH2) | 3.04 | 19.8 |
| Thr126 (Oα) | Meropenem (NH2) | 2.78 | 45.4 |
For the case of Meropenem, it can interact with almost the same residues as Imipenem. As can be seen from Fig. 3C, the binding pocket for Meropenem involves six residues, of which Glu88 and Thr126 form three hydrogen bonds with the antibiotic drug. Since there is one more five-member ring in Meropenem (Fig. 3C), some additional hydrogen bonds are needed to stabilize the ligand during the process of cleaving its amide bond and inactivate the antibiotic drug. Based on these structural findings and previous theoretical studies [56], [57], [58], we proposed a catalytic mechanism for NDM-1, as illustrated in Fig. 4. In our model, the metal-binding Asp60 acts as the general base that activates the water nucleophile, while the protonation of Asp60 results in the cleavage of its bond to the metal ion.
Supporting Information
Ramachandran plot for the computational model of NDM-1 by PROCHECK.
(TIF)
The density plot of the QMEAN score for the computational model of NDM-1.
(TIF)
Estimated absolute model quality by the comparison of the QMEAN scores with the reference x-ray structures in the Protein Data Bank.
(TIF)
The QMEAN score components calculated based on the Z-score of each component in comparison with the average x-ray structures.
(TIF)
Per-residue error visualized by using a color gradient from blue (reliable region, estimated error below 1 Å) to red (potentially unreliable regions, estimated error above 3.5 Å).
(TIF)
Acknowledgments
The authors are very much indebted to Dr. Naoyuki Taniguchi for his constructive comments, which are very helpful for strengthening the presentation of this paper.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.Giamarellou H, Poulakou G. Multidrug-resistant Gram-negative infections: what are the treatment options? Drugs. 2009;69:1879–1901. doi: 10.2165/11315690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Livermore DM. beta-Lactamases- the Threat Renews. Curr Protein Pept Sci. 2009;10:397–400. doi: 10.2174/138920309789351994. [DOI] [PubMed] [Google Scholar]
- 3.Maltezou HC. Metallo-beta-lactamases in Gram-negative bacteria: introducing the era of pan-resistance? Int J Antimicrob Agents. 2009;33:e401–407. doi: 10.1016/j.ijantimicag.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53:5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poirel L, Lagrutta E, Taylor P, Pham J, Nordmann P. Emergence of metallo-ss-lactamase NDM-1-producing multidrug resistant Escherichia coli in Australia. Antimicrob Agents Chemother. 2010 doi: 10.1128/AAC.00878-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou KC. Review: Structural bioinformatics and its impact to biomedical science. Current Medicinal Chemistry. 2004;11:2105–2134. doi: 10.2174/0929867043364667. [DOI] [PubMed] [Google Scholar]
- 8.Wang JF, Wei DQ, Li L, Zheng SY, Li YX, et al. 3D structure modeling of cytochrome P450 2C19 and its implication for personalized drug design. Biochem Biophys Res Commun. 2007;355:513–519. doi: 10.1016/j.bbrc.2007.01.185. Erratum in: Biochem Biophys Res Commun 2007, 357: 339; Biochem Biophys Res Commun 2009, 384: 399. [DOI] [PubMed] [Google Scholar]
- 9.Chou KC. Modelling extracellular domains of GABA-A receptors: subtypes 1, 2, 3, and 5. Biochemical and Biophysical Research Communications. 2004;316:636–642. doi: 10.1016/j.bbrc.2004.02.098. [DOI] [PubMed] [Google Scholar]
- 10.Wang JF, Wei DQ, Lin Y, Wang YH, Du HL, et al. Insights from modeling the 3D structure of NAD(P)H-dependent D-xylose reductase of Pichia stipitis and its binding interactions with NAD and NADP. Biochem Biophys Res Commun. 2007;359:323–329. doi: 10.1016/j.bbrc.2007.05.101. [DOI] [PubMed] [Google Scholar]
- 11.Chou KC. Insights from modelling the 3D structure of the extracellular domain of alpha7 nicotinic acetylcholine receptor. Biochemical and Biophysical Research Communication. 2004;319:433–438. doi: 10.1016/j.bbrc.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Wang JF, Wei DQ, Chen C, Li Y, Chou KC. Molecular modeling of two CYP2C19 SNPs and its implications for personalized drug design. Protein Pept Lett. 2008;15:27–32. doi: 10.2174/092986608783330305. [DOI] [PubMed] [Google Scholar]
- 13.Chou KC. Molecular therapeutic target for type-2 diabetes. Journal of Proteome Research. 2004;3:1284–1288. doi: 10.1021/pr049849v. [DOI] [PubMed] [Google Scholar]
- 14.Wang JF, Wei DQ, Chou KC. Pharmacogenomics and personalized use of drugs. Curr Top Med Chem. 2008;8:1573–1579. doi: 10.2174/156802608786786534. [DOI] [PubMed] [Google Scholar]
- 15.Chou KC. Coupling interaction between thromboxane A2 receptor and alpha-13 subunit of guanine nucleotide-binding protein. Journal of Proteome Research. 2005;4:1681–1686. doi: 10.1021/pr050145a. [DOI] [PubMed] [Google Scholar]
- 16.Wang JF, Chou KC. Insight into the molecular switch mechanism of human Rab5a from molecular dynamics simulations. Biochem Biophys Res Commun. 2009;390:608–612. doi: 10.1016/j.bbrc.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Gong K, Li L, Wang JF, Cheng F, Wei DQ, et al. Binding mechanism of H5N1 influenza virus neuraminidase with ligands and its implication for drug design. Med Chem. 2009;5:242–249. doi: 10.2174/157340609788185936. [DOI] [PubMed] [Google Scholar]
- 18.Gu RX, Gu H, Xie ZY, Wang JF, Arias HR, et al. Possible drug candidates for Alzheimer's disease deduced from studying their binding interactions with alpha7 nicotinic acetylcholine receptor. Med Chem. 2009;5:250–262. doi: 10.2174/157340609788185909. [DOI] [PubMed] [Google Scholar]
- 19.Chou KC, Wei DQ, Zhong WZ. Binding mechanism of coronavirus main proteinase with ligands and its implication to drug design against SARS. Biochem Biophys Res Comm. 2003;308:148–151. doi: 10.1016/S0006-291X(03)01342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang JF, Gong K, Wei DQ, Li YX, Chou KC. Molecular dynamics studies on the interactions of PTP1B with inhibitors: from the first phosphate-binding site to the second one. Protein Eng Des Sel. 2009;22:349–355. doi: 10.1093/protein/gzp012. [DOI] [PubMed] [Google Scholar]
- 21.Zeng QK, Du HL, Wang JF, Wei DQ, Wang XN, et al. Reversal of coenzyme specificity and improvement of catalytic efficiency of Pichia stipitis xylose reductase by rational site-directed mutagenesis. Biotechnol Lett. 2009;31:1025–1029. doi: 10.1007/s10529-009-9980-x. [DOI] [PubMed] [Google Scholar]
- 22.Wang JF, Chou KC. Molecular modeling of cytochrome P450 and drug metabolism. Curr Drug Metab. 2010;11:342–346. doi: 10.2174/138920010791514180. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Wei DQ, Wang JF, Chou KC. Computational studies of the binding mechanism of calmodulin with chrysin. Biochem Biophys Res Commun. 2007;358:1102–1107. doi: 10.1016/j.bbrc.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 24.Wang JF, Yan JY, Wei DQ, Chou KC. Binding of CYP2C9 with diverse drugs and its implications for metabolic mechanism. Med Chem. 2009;5:263–270. doi: 10.2174/157340609788185954. [DOI] [PubMed] [Google Scholar]
- 25.Wang JF, Wei DQ. Role of structural bioinformatics and traditional Chinese medicine databases in pharmacogenomics. Pharmacogenomics. 2009;10:1213–1215. doi: 10.2217/pgs.09.81. [DOI] [PubMed] [Google Scholar]
- 26.Wang JF, Zhang CC, Chou KC, Wei DQ. Structure of cytochrome p450s and personalized drug. Curr Med Chem. 2009;16:232–244. doi: 10.2174/092986709787002727. [DOI] [PubMed] [Google Scholar]
- 27.Gui H, Chen HF, Wei DQ, Wang JF. Molecular dynamics simulations exploring drug resistance in HIV-1 proteases. Chinese Sci Bull. 2010;55:2677–2683. [Google Scholar]
- 28.Tang B, Gong K, Wang JF, Li YX, Wei DQ. The structure of phospholamban and its MD simulations. Chinese Sci Bull. 2010;55:1619–1624. [Google Scholar]
- 29.Wang JF, Gong K, Wei DQ, Li YX. Structural flexibility and interactions of PTP1B's S-loop. Interdiscip Sci. 2009;1:214–219. doi: 10.1007/s12539-009-0047-4. [DOI] [PubMed] [Google Scholar]
- 30.Wang JF, Chou KC. Insight from studying the mutation-induced allostery in the M2 proton channel by molecular dynamics. Protein Eng Des Sel. 2010;23:663–666. doi: 10.1093/protein/gzq040. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Wei DQ, Wang JF. Molecular dynamics studies on T1 lipase: insights into a double-flap mechanism. J Chem Inf Model. 2010;50:875–878. doi: 10.1021/ci900458u. [DOI] [PubMed] [Google Scholar]
- 32.Wang JF, Wei DQ, Du HL, Li YX, Chou KC. Molecular modeling studies on NADP-dependence of candida tropicalis strain xylose reductase. Open Bioinformatics J. 2008;2:72–79. [Google Scholar]
- 33.Huang RB, Du QS, Wang CH, Chou KC. An in-depth analysis of the biological functional studies based on the NMR M2 channel structure of influenza A virus. Biochem Biophys Res Comm. 2008;377:1243–1247. doi: 10.1016/j.bbrc.2008.10.148. [DOI] [PubMed] [Google Scholar]
- 34.Du QS, Huang RB, Wang CH, Li XM, Chou KC. Energetic analysis of the two controversial drug binding sites of the M2 proton channel in influenza A virus. Journal of Theoretical Biology. 2009;259:159–164. doi: 10.1016/j.jtbi.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Wei H, Wang CH, Du QS, Meng J, Chou KC. Investigation into adamantane-based M2 inhibitors with FB-QSAR. Medicinal Chemistry. 2009;5:305–317. doi: 10.2174/157340609788681430. [DOI] [PubMed] [Google Scholar]
- 36.Wang JF, Wei DQ, Chou KC. Insights from investigating the interactions of adamantane-based drugs with the M2 proton channel from the H1N1 swine virus. Biochem Biophys Res Commun. 2009;388:413–417. doi: 10.1016/j.bbrc.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 37.Guo X, Wang JF, Zhu Y, Wei DQ. Recent Progress on Computer-Aided Inhibitor Design of H5N1 Influenza A Virus. Curr Comput Aided Drug Des. 2010;6:139–146. doi: 10.2174/157340910791202487. [DOI] [PubMed] [Google Scholar]
- 38.Chou KC, Wei DQ, Du QS, Sirois S, Zhong WZ. Review: Progress in computational approach to drug development against SARS. Current Medicinal Chemistry. 2006;13:3263–3270. doi: 10.2174/092986706778773077. [DOI] [PubMed] [Google Scholar]
- 39.Du QS, Huang RB, Wang SQ, Chou KC. Designing inhibitors of M2 proton channel against H1N1 swine influenza virus. PLoS ONE. 2010;5:e9388. doi: 10.1371/journal.pone.0009388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Saez I, Docquier JD, Rossolini GM, Dideberg O. The three-dimensional structure of VIM-2, a Zn-beta-lactamase from Pseudomonas aeruginosa in its reduced and oxidised form. J Mol Biol. 2008;375:604–611. doi: 10.1016/j.jmb.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 41.Shen HB, Chou KC. EzyPred: A top-down approach for predicting enzyme functional classes and subclasses. Biochem Biophys Res Comm. 2007;364:53–59. doi: 10.1016/j.bbrc.2007.09.098. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y. Template-based modeling and free modeling by I-TASSER in CASP7. Proteins. 2007;69(Suppl 8):108–117. doi: 10.1002/prot.21702. [DOI] [PubMed] [Google Scholar]
- 44.Wu S, Skolnick J, Zhang Y. Ab initio modeling of small proteins by iterative TASSER simulations. BMC Biol. 2007;5:17. doi: 10.1186/1741-7007-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chou KC, Carlacci L. Simulated annealing approach to the study of protein structures. Protein Engineering. 1991;4:661–667. doi: 10.1093/protein/4.6.661. [DOI] [PubMed] [Google Scholar]
- 46.Laurie AT, Jackson RM. Q-SiteFinder: an energy-based method for the prediction of protein-ligand binding sites. Bioinformatics. 2005;21:1908–1916. doi: 10.1093/bioinformatics/bti315. [DOI] [PubMed] [Google Scholar]
- 47.Concha NO, Janson CA, Rowling P, Pearson S, Cheever CA, et al. Crystal structure of the IMP-1 metallo beta-lactamase from Pseudomonas aeruginosa and its complex with a mercaptocarboxylate inhibitor: binding determinants of a potent, broad-spectrum inhibitor. Biochemistry. 2000;39:4288–4298. doi: 10.1021/bi992569m. [DOI] [PubMed] [Google Scholar]
- 48.Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, et al. Automated dokcing using a lamarckian genetic algorithm and empirical binding free energy function. J Comput Chem 1998. 1998;19:1639–1662. Journal of Computational Chemistry 19: 1639–1662. [Google Scholar]
- 49.Colotta V, Capelli F, Lenzi O, Catarzi D, Varano F, et al. Novel potent and highly selective human A(3) adenosine receptor antagonists belonging to the 4-amido-2-arylpyrazolo[3,4-c]quinoline series: molecular docking analysis and pharmacological studies. Bioorg Med Chem. 2009;17:401–410. doi: 10.1016/j.bmc.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 50.Ricchiuto P, Rocco AG, Gianazza E, Corrada D, Beringhelli T, et al. Structural and dynamic roles of permanent water molecules in ligand molecular recognition by chicken liver bile acid binding protein. J Mol Recognit. 2008;21:348–354. doi: 10.1002/jmr.908. [DOI] [PubMed] [Google Scholar]
- 51.Magdziarz T, Mazur P, Polanski J. Receptor independent and receptor dependent CoMSA modeling with IVE-PLS: application to CBG benchmark steroids and reductase activators. J Mol Model. 2009;15:41–51. doi: 10.1007/s00894-008-0373-1. [DOI] [PubMed] [Google Scholar]
- 52.Laskowski RA, MacArthur MW, Moss D, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Cryst. 1993;26:283–291. [Google Scholar]
- 53.Benkert P, Tosatto SCE, Schomburg D. QMEAN: A comprehensive scoring function for model quality assessment. Proteins. 2008;71:261–277. doi: 10.1002/prot.21715. [DOI] [PubMed] [Google Scholar]
- 54.Chou KC, Zhang CT. Review: Prediction of protein structural classes. Critical Reviews in Biochemistry and Molecular Biology. 1995;30:275–349. doi: 10.3109/10409239509083488. [DOI] [PubMed] [Google Scholar]
- 55.Chou KC, Watenpaugh KD, Heinrikson RL. A Model of the complex between cyclin-dependent kinase 5 (Cdk5) and the activation domain of neuronal Cdk5 activator. Biochem Biophys Res Commun. 1999;259:420–428. doi: 10.1006/bbrc.1999.0792. [DOI] [PubMed] [Google Scholar]
- 56.Wang Z, Fast W, Valentine AM, Benkovic SJ. Metallo-beta-lactamase: structure and mechanism. Curr Opin Chem Biol. 1999;3:614–622. doi: 10.1016/s1367-5931(99)00017-4. [DOI] [PubMed] [Google Scholar]
- 57.Xu D, Guo H, Cui Q. Antibiotic deactivation by a dizinc beta-lactamase: mechanistic insights from QM/MM and DFT studies. J Am Chem Soc. 2007;129:10814–10822. doi: 10.1021/ja072532m. [DOI] [PubMed] [Google Scholar]
- 58.Tamilselvi A, Nethaji M, Mugesh G. Antibiotic resistance: mono- and dinuclear zinc complexes as metallo-beta-lactamase mimics. Chemistry. 2006;12:7797–7806. doi: 10.1002/chem.200600629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ramachandran plot for the computational model of NDM-1 by PROCHECK.
(TIF)
The density plot of the QMEAN score for the computational model of NDM-1.
(TIF)
Estimated absolute model quality by the comparison of the QMEAN scores with the reference x-ray structures in the Protein Data Bank.
(TIF)
The QMEAN score components calculated based on the Z-score of each component in comparison with the average x-ray structures.
(TIF)
Per-residue error visualized by using a color gradient from blue (reliable region, estimated error below 1 Å) to red (potentially unreliable regions, estimated error above 3.5 Å).
(TIF)