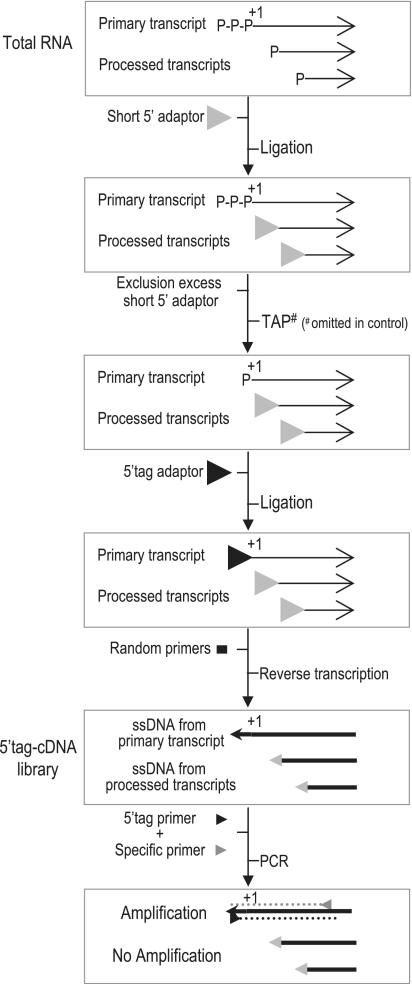
Figure 1.
Principle of the 5′tagRACE method. 5′ monophosphate RNA extremities generated by processing or degradation are ligated to an excess of a 5′ RNA adaptor (‘Short 5′ adaptor’), resulting in inactive 5′-ends for subsequent ligation steps. The excess of 5′ RNA adaptor is eliminated via exclusion chromatography. 5′ triphosphate ends of primary transcripts (i.e. ‘+1’ or ‘TSS’) are then transformed into 5′ monophosphate by the TAP enzyme and ligated to a second 5′ RNA adaptor (‘5′tag adaptor’). Reverse transcription is performed using random primers to generate a cDNA library (5′tag-cDNA library) containing two types of 5′tagged single-stranded-DNA molecules: those ligated to the first 5′ adaptor (Short 5′ adaptor), i.e. processed ends, and those ligated to the 5′ tag adaptor, i.e. TSSs. The use of a specific primer to the 5′ tag adaptor and an oligonucleotide specific to the selected RNA (transformed into cDNA) will allow the specific amplification by PCR of the cDNA synthesized only from the primary RNA. An untreated TAP 5′tag-cDNA library is used as negative control (hash sign).