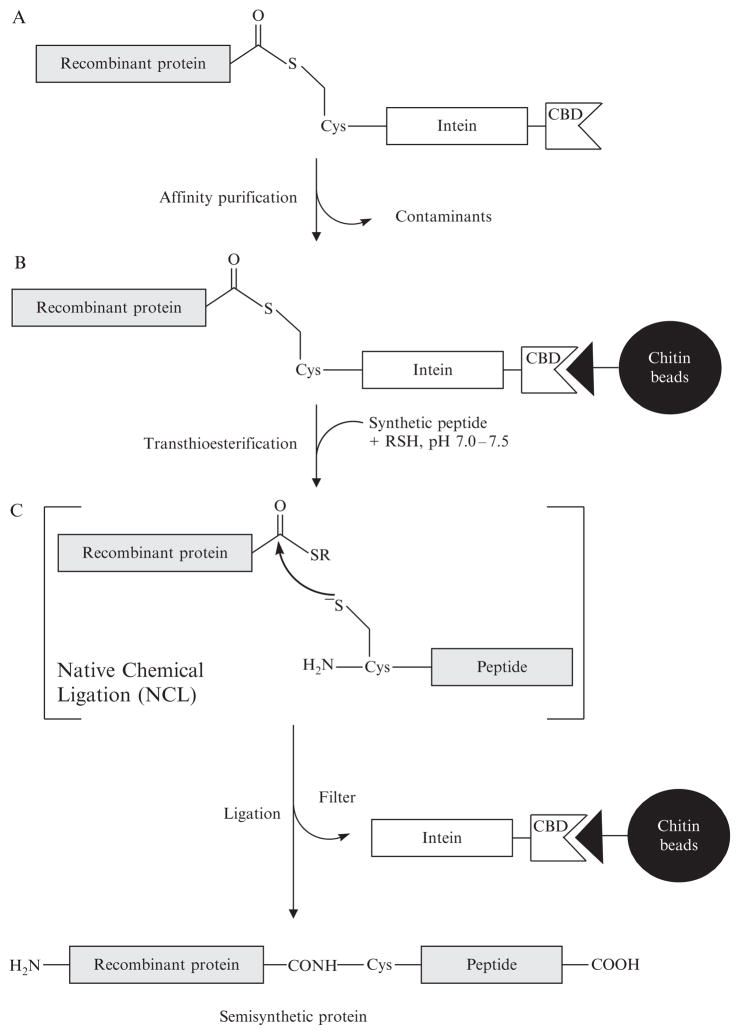
Figure 1.2.
Expressed protein ligation. (A) Recombinant protein for EPL is expressed as a C-terminal intein-CBD fusion protein and subjected to affinity purification on chitin beads. The modified inteins used for semisynthesis catalyze an N- to S-acyl shift resulting in the formation of a thioester-linked fusion protein. (B) The protein thioester is trapped by small-molecule thiol (RSH = sodium 2-mercaptoethansulfonate, thiophenol, or mercaptophenylacetic acid) as a more reactive αthioester for semisynthesis. (C) In the native chemical ligation reaction, the αthioester intermediate is intercepted by a 1Cys synthetic peptide and the newly formed thioester-linked species spontaneously rearranges to yield a native peptide bond at pH 7.0–7.5. The semisynthetic protein is eluted from the column and now contains a chemoselectively ligated synthetic peptide at the C-terminus.