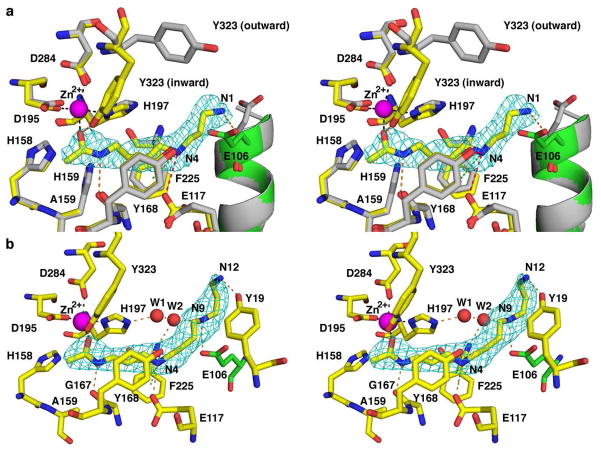
Figure 9.
Acetylpolyamine binding in the active site of H159A APAH. Atoms are colorcoded as follows: C = yellow (H159A APAH-N8-acetylspermidine complex), green (adjacent monomer in H159A APAH-N8-acetylspermidine complex), or gray (APAHCAPS complex); N = blue; O = red; Zn2+ = magenta sphere; water molecules are shown as red spheres. (A) Simulated annealing omit map of N8-acetylspermidine contoured at 4.5 σ. Metal coordination, hydrogen bond, electrostatic, and cation-π interactions are shown as black, orange, pink, and green dashed lines, respectively. The side chain of E106 (green) is from the adjacent monomer. Conformational changes of Y323, E117, and E106 accommodate substrate binding as compared with the wild-type enzyme complexed with CAPS (gray). (B) Simulated annealing omit map of acetylspermine contoured at 5 σ.