Abstract
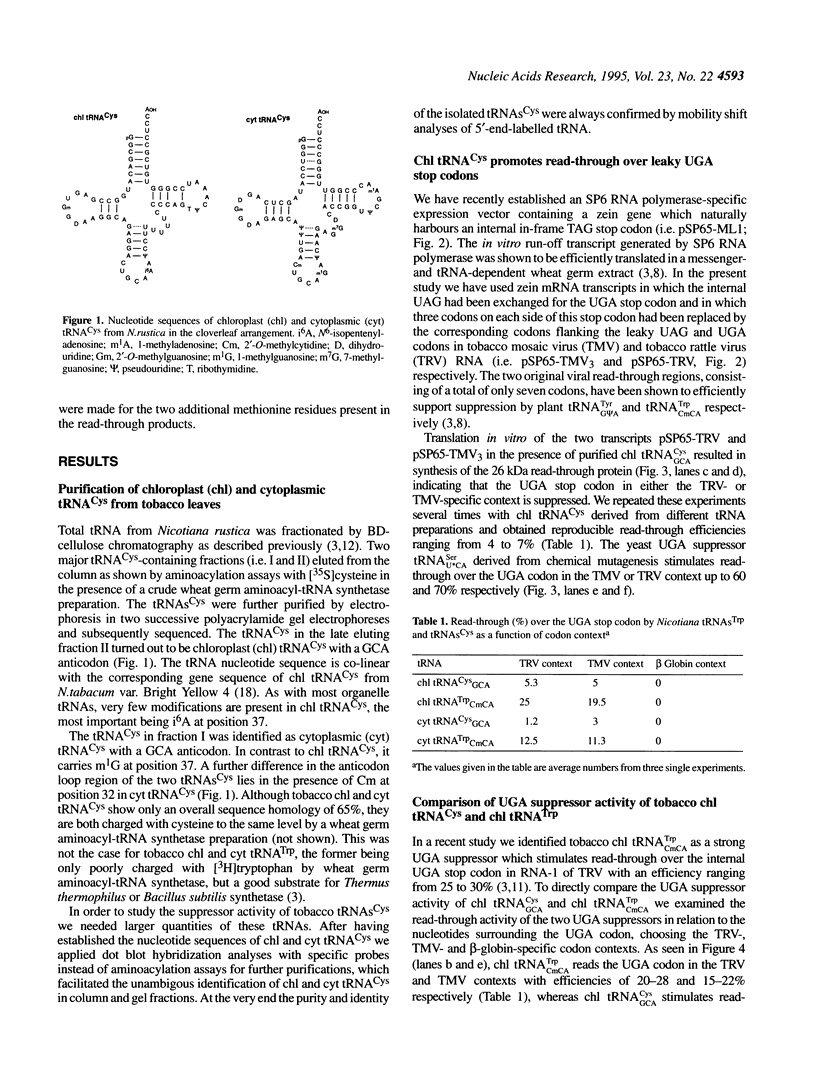
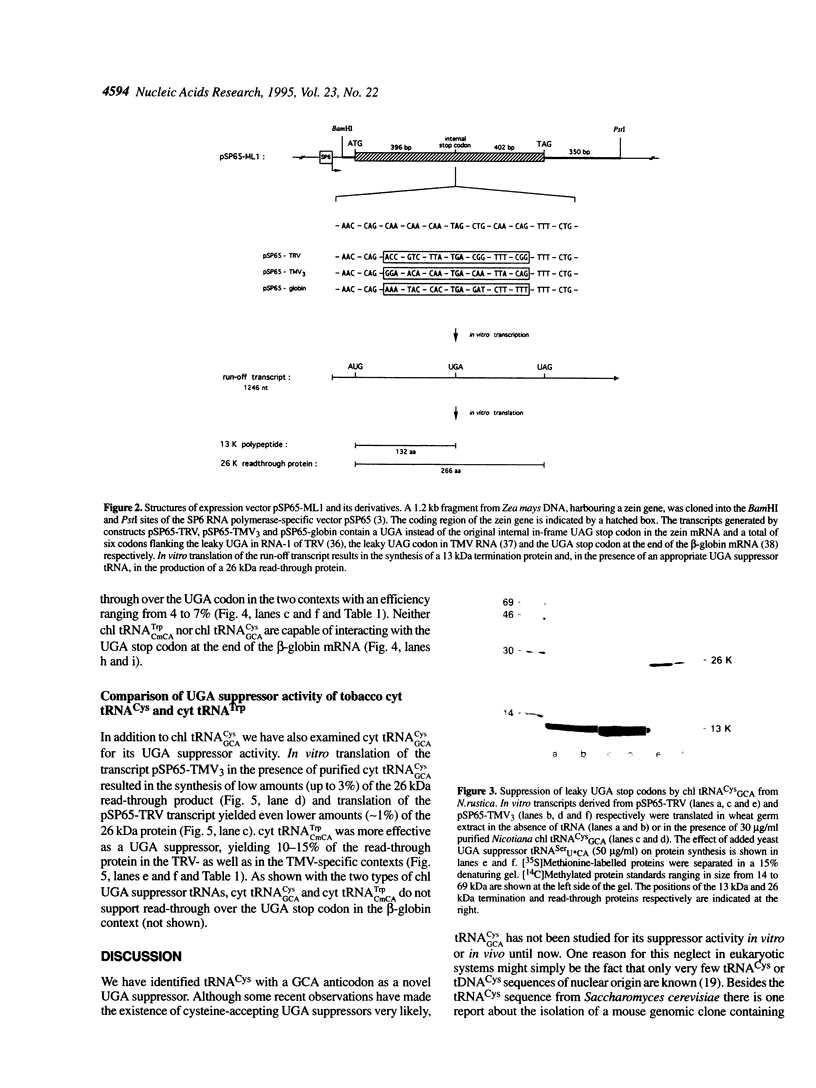
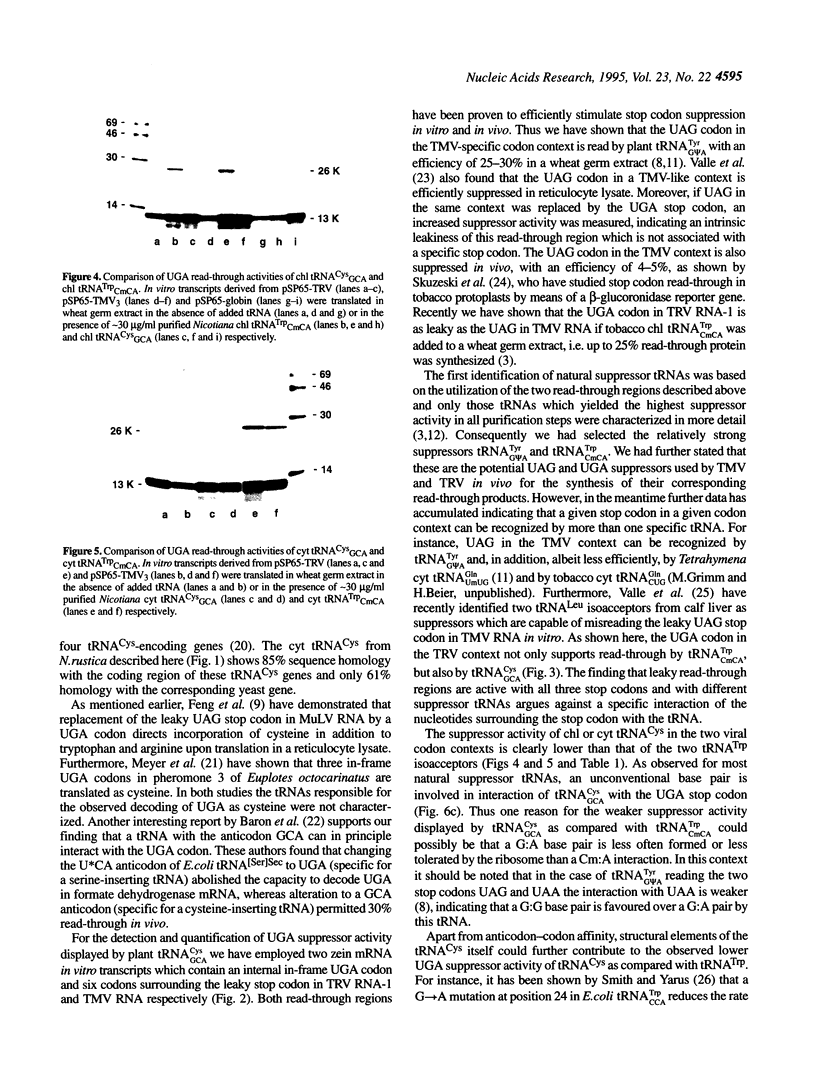
We have isolated and sequenced chloroplast (chl) and cytoplasmic (cyt) cysteine tRNAs from Nicotiana rustica. Both tRNAs carry a GCA anticodon but beyond that differ considerably in their nucleotide sequences. One obvious distinction resides in the presence of N6-isopentenyladenosine (i6A) and 1-methylguanosine (m1G) at position 37 in chl and cyt tRNA(Cys) respectively. In order to study the potential suppressor activity of tRNAs(Cys) we used in vitro synthesized zein mRNA transcripts in which an internal UGA stop codon had been placed in either the tobacco rattle virus (TRV)- or tobacco mosaic virus (TMV)-specific codon context. In vitro translation was carried out in a messenger- and tRNA-dependent wheat germ extract. Both tRNA(Cys) isoacceptors stimulate read-through over the UGA stop codon, however, chl tRNA(GCA)Cys is more efficient than the cytoplasmic counterpart. The UGA in the two viral codon contexts is suppressed to about the same extent by either of the two tRNAs(Cys), whereas UGA in the beta-globin context is not recognized at all. The interaction of tRNA(GCA)Cys with UGA requires an unconventional G:A base pair in the wobble position, as postulated earlier for plant tRNA(G psi A)Tyr misreading the UAA stop codon. This is the first case that a cysteine-accepting tRNA has been characterized as a natural UGA suppressor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amberg R., Urban C., Reuner B., Scharff P., Pomerantz S. C., McCloskey J. A., Gross H. J. Editing does not exist for mammalian selenocysteine tRNAs. Nucleic Acids Res. 1993 Dec 11;21(24):5583–5588. doi: 10.1093/nar/21.24.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron C., Heider J., Böck A. Mutagenesis of selC, the gene for the selenocysteine-inserting tRNA-species in E. coli: effects on in vivo function. Nucleic Acids Res. 1990 Dec 11;18(23):6761–6766. doi: 10.1093/nar/18.23.6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier H., Barciszewska M., Krupp G., Mitnacht R., Gross H. J. UAG readthrough during TMV RNA translation: isolation and sequence of two tRNAs with suppressor activity from tobacco plants. EMBO J. 1984 Feb;3(2):351–356. doi: 10.1002/j.1460-2075.1984.tb01810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk G. R., Wikström P. M., Byström A. S. Prevention of translational frameshifting by the modified nucleoside 1-methylguanosine. Science. 1989 May 26;244(4907):986–989. doi: 10.1126/science.2471265. [DOI] [PubMed] [Google Scholar]
- Buckingham R. H. Codon context and protein synthesis: enhancements of the genetic code. Biochimie. 1994;76(5):351–354. doi: 10.1016/0300-9084(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Böck A., Forchhammer K., Heider J., Baron C. Selenoprotein synthesis: an expansion of the genetic code. Trends Biochem Sci. 1991 Dec;16(12):463–467. doi: 10.1016/0968-0004(91)90180-4. [DOI] [PubMed] [Google Scholar]
- Demler S. A., de Zoeten G. A. The nucleotide sequence and luteovirus-like nature of RNA 1 of an aphid non-transmissible strain of pea enation mosaic virus. J Gen Virol. 1991 Aug;72(Pt 8):1819–1834. doi: 10.1099/0022-1317-72-8-1819. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maniatis T. The primary structure of rabbit beta-globin mRNA as determined from cloned DNA. Cell. 1977 Apr;10(4):571–585. doi: 10.1016/0092-8674(77)90090-3. [DOI] [PubMed] [Google Scholar]
- Ericson J. U., Björk G. R. tRNA anticodons with the modified nucleoside 2-methylthio-N6-(4-hydroxyisopentenyl)adenosine distinguish between bases 3' of the codon. J Mol Biol. 1991 Apr 5;218(3):509–516. doi: 10.1016/0022-2836(91)90697-5. [DOI] [PubMed] [Google Scholar]
- Farabaugh P. J. Alternative readings of the genetic code. Cell. 1993 Aug 27;74(4):591–596. doi: 10.1016/0092-8674(93)90507-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y. X., Copeland T. D., Oroszlan S., Rein A., Levin J. G. Identification of amino acids inserted during suppression of UAA and UGA termination codons at the gag-pol junction of Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8860–8863. doi: 10.1073/pnas.87.22.8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelet P., Lomonossoff G. P., Butler P. J., Akam M. E., Gait M. J., Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5818–5822. doi: 10.1073/pnas.79.19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagervall T. G., Ericson J. U., Esberg K. B., Li J. N., Björk G. R. Role of tRNA modification in translational fidelity. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):263–266. doi: 10.1016/0167-4781(90)90178-5. [DOI] [PubMed] [Google Scholar]
- Hamilton W. D., Boccara M., Robinson D. J., Baulcombe D. C. The complete nucleotide sequence of tobacco rattle virus RNA-1. J Gen Virol. 1987 Oct;68(Pt 10):2563–2575. doi: 10.1099/0022-1317-68-10-2563. [DOI] [PubMed] [Google Scholar]
- Hatfield D. L., Smith D. W., Lee B. J., Worland P. J., Oroszlan S. Structure and function of suppressor tRNAs in higher eukaryotes. Crit Rev Biochem Mol Biol. 1990;25(2):71–96. doi: 10.3109/10409239009090606. [DOI] [PubMed] [Google Scholar]
- Heitzler J., Maréchal-Drouard L., Dirheimer G., Keith G. Use of a dot blot hybridization method for identification of pure tRNA species on different membranes. Biochim Biophys Acta. 1992 Feb 11;1129(3):273–277. doi: 10.1016/0167-4781(92)90503-r. [DOI] [PubMed] [Google Scholar]
- Herzog E., Guilley H., Manohar S. K., Dollet M., Richards K., Fritsch C., Jonard G. Complete nucleotide sequence of peanut clump virus RNA 1 and relationships with other fungus-transmitted rod-shaped viruses. J Gen Virol. 1994 Nov;75(Pt 11):3147–3155. doi: 10.1099/0022-1317-75-11-3147. [DOI] [PubMed] [Google Scholar]
- Hirsh D., Gold L. Translation of the UGA triplet in vitro by tryptophan transfer RNA's. J Mol Biol. 1971 Jun 14;58(2):459–468. doi: 10.1016/0022-2836(71)90363-9. [DOI] [PubMed] [Google Scholar]
- MacFarlane S. A., Taylor S. C., King D. I., Hughes G., Davies J. W. Pea early browning virus RNA1 encodes four polypeptides including a putative zinc-finger protein. Nucleic Acids Res. 1989 Mar 25;17(6):2245–2260. doi: 10.1093/nar/17.6.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer F., Schmidt H. J., Plümper E., Hasilik A., Mersmann G., Meyer H. E., Engström A., Heckmann K. UGA is translated as cysteine in pheromone 3 of Euplotes octocarinatus. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3758–3761. doi: 10.1073/pnas.88.9.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottagui-Tabar S., Björnsson A., Isaksson L. A. The second to last amino acid in the nascent peptide as a codon context determinant. EMBO J. 1994 Jan 1;13(1):249–257. doi: 10.1002/j.1460-2075.1994.tb06255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutter R. C., Scheets K., Panganiban L. C., Lommel S. A. The complete nucleotide sequence of the maize chlorotic mottle virus genome. Nucleic Acids Res. 1989 Apr 25;17(8):3163–3177. doi: 10.1093/nar/17.8.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafalski A., Kohli J., Agris P., Söll D. The nucleotide sequence of a UGA suppressor serine tRNA from Schizosaccharomyces pombe. Nucleic Acids Res. 1979 Jun 25;6(8):2683–2695. doi: 10.1093/nar/6.8.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde W., Gramstat A., Schmitz J., Tacke E., Prüfer D. Plant viruses as model systems for the study of non-canonical translation mechanisms in higher plants. J Gen Virol. 1994 Sep;75(Pt 9):2141–2149. doi: 10.1099/0022-1317-75-9-2141. [DOI] [PubMed] [Google Scholar]
- Schüll C., Beier H. Three Tetrahymena tRNA(Gln) isoacceptors as tools for studying unorthodox codon recognition and codon context effects during protein synthesis in vitro. Nucleic Acids Res. 1994 Jun 11;22(11):1974–1980. doi: 10.1093/nar/22.11.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirako Y., Wilson T. M. Complete nucleotide sequence and organization of the bipartite RNA genome of soil-borne wheat mosaic virus. Virology. 1993 Jul;195(1):16–32. doi: 10.1006/viro.1993.1342. [DOI] [PubMed] [Google Scholar]
- Skuzeski J. M., Nichols L. M., Gesteland R. F., Atkins J. F. The signal for a leaky UAG stop codon in several plant viruses includes the two downstream codons. J Mol Biol. 1991 Mar 20;218(2):365–373. doi: 10.1016/0022-2836(91)90718-l. [DOI] [PubMed] [Google Scholar]
- Smith D., Yarus M. Transfer RNA structure and coding specificity. I. Evidence that a D-arm mutation reduces tRNA dissociation from the ribosome. J Mol Biol. 1989 Apr 5;206(3):489–501. doi: 10.1016/0022-2836(89)90496-8. [DOI] [PubMed] [Google Scholar]
- Stanley J., Vassilenko S. A different approach to RNA sequencing. Nature. 1978 Jul 6;274(5666):87–89. doi: 10.1038/274087a0. [DOI] [PubMed] [Google Scholar]
- Steinberg S., Misch A., Sprinzl M. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1993 Jul 1;21(13):3011–3015. doi: 10.1093/nar/21.13.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuite M. F., Stansfield I. Termination of protein synthesis. Mol Biol Rep. 1994 May;19(3):171–181. doi: 10.1007/BF00986959. [DOI] [PubMed] [Google Scholar]
- Vacher J., Grosjean H., de Henau S., Finelli J., Buckingham R. H. Construction of a UGA suppressor tRNA by modification in vitro of yeast tRNACys. Eur J Biochem. 1984 Jan 2;138(1):77–81. doi: 10.1111/j.1432-1033.1984.tb07883.x. [DOI] [PubMed] [Google Scholar]
- Valle R. P., Drugeon G., Devignes-Morch M. D., Legocki A. B., Haenni A. L. Codon context effect in virus translational readthrough. A study in vitro of the determinants of TMV and Mo-MuLV amber suppression. FEBS Lett. 1992 Jul 20;306(2-3):133–139. doi: 10.1016/0014-5793(92)80984-o. [DOI] [PubMed] [Google Scholar]
- Valle R. P., Morch M. D., Haenni A. L. Novel amber suppressor tRNAs of mammalian origin. EMBO J. 1987 Oct;6(10):3049–3055. doi: 10.1002/j.1460-2075.1987.tb02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle R. P., Morch M. D. Stop making sense: or Regulation at the level of termination in eukaryotic protein synthesis. FEBS Lett. 1988 Aug 1;235(1-2):1–15. doi: 10.1016/0014-5793(88)81225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandelt C., Feix G. Sequence of a 21 kd zein gene from maize containing an in-frame stop codon. Nucleic Acids Res. 1989 Mar 25;17(6):2354–2354. doi: 10.1093/nar/17.6.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. M., Weber K. Natural read-through at the UGA termination signal of Q-beta coat protein cistron. Nat New Biol. 1971 Sep 15;234(50):206–209. doi: 10.1038/newbio234206a0. [DOI] [PubMed] [Google Scholar]
- Wood L., Hatzenbuhler N., Peterson R., Vogeli G. Isolation of a mouse genomic clone containing four tRNACys-encoding genes. Gene. 1991 Feb 15;98(2):249–252. doi: 10.1016/0378-1119(91)90181-a. [DOI] [PubMed] [Google Scholar]
- Zerfass K., Beier H. Pseudouridine in the anticodon G psi A of plant cytoplasmic tRNA(Tyr) is required for UAG and UAA suppression in the TMV-specific context. Nucleic Acids Res. 1992 Nov 25;20(22):5911–5918. doi: 10.1093/nar/20.22.5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerfass K., Beier H. The leaky UGA termination codon of tobacco rattle virus RNA is suppressed by tobacco chloroplast and cytoplasmic tRNAs(Trp) with CmCA anticodon. EMBO J. 1992 Nov;11(11):4167–4173. doi: 10.1002/j.1460-2075.1992.tb05510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]