Abstract
The management of high-dose methotrexate (MTX) therapy in patients with cancer depends on the routine monitoring of drug exposures in conjunction with leucovorin (LV), urine pH, patient hydration and other clinical indices of patient well-being. A key factor in patient oversight is the facilitation of MTX clearance in order to minimize drug-related toxicity. The aim of this investigation was to evaluate the performance of a clinical decision support system and Bayesian forecasting algorithm in the prediction of MTX concentrations and assessment of LV dosing requirements in pediatric and young adult cancer patients based on the current practice at the Children’s Hospital of Philadelphia. Fifty patients ranging in age from 8 months to 21 years (weight range,7.6 to 163.3 kg) contributing 80 total dosing events (183 MTX serum concentrations) were studied. The forecasting model was able to consistently predict future MTX concentrations with the knowledge of one prior concentration and continued to improve with additional concentration data made available through daily therapeutic drug monitoring. Precision was good at 12.9% with low bias at 2.2%. Comparison between the decision support system recommendations for LV rescue relative to the actual LV administration was also made. Sixteen patients would have initiated rescue therapy earlier, 7 patients would have received a larger dose (42 smaller) and LV would have been given less often for 37 patients. The forecasting algorithm in the MTX dashboard was reasonably accurate in predicting MTX concentrations and should improve further as the underlying model and prediction algorithm evolves. This decision support system can be useful in helping physicians decide if a patient is clearing MTX as expected or if more aggressive rescue therapy is warranted.
Keywords: methotrexate, leucovorin, therapeutic drug monitoring, decision support system, pediatric oncology
INTRODUCTION
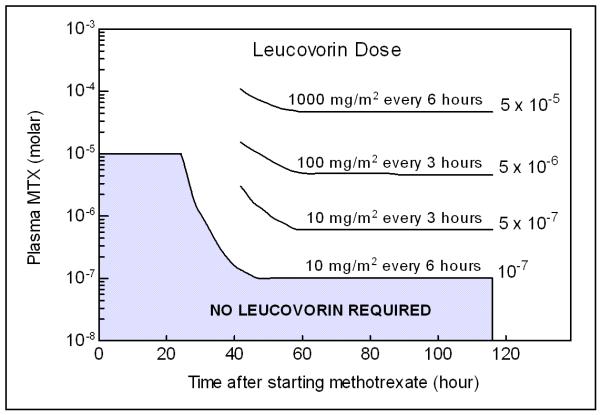
Methotrexate (MTX) is an antifolate agent, inhibiting the metabolism of folic acid by binding to dihydrofolate reductase, used in the treatment of various cancers including acute lymphoblastic leukemia (ALL), non-Hodgkin Lymphoma (NHL) and osteosarcoma. It ranks second in overall utilization among oncology agents at the Children’s Hospital of Philadelphia1 and is highly utilized worldwide in these cancer indications. One difficultly facing physicians when administering high-dose MTX (HDMTX, > 1 g/m2) is balancing its safety and efficacy. MTX removal from plasma after intraveneous injection occurs in a triphasic manner. Plasma concentrations are typically between 10−2 to 10−4 M with an exponential decay. MTX is metabolized into 7-hydroxy-methotrexate (7-OH-MTX) and 2,4-diamino-N10-methylpteroic acid (DAMPA), an inactive metabolite.2 MTX is primarily excreted through the kidneys and renal dysfunction can result in the delayed excretion of MTX during the third phase. Both high exposure over short durations and low exposure for prolonged duration can lead to toxicity. MTX can be tolerated in doses ranging from 900 to 30000 mg/m2 if it is given along with LV rescue therapy.3 Toxicity plasma concentration thresholds vary based on organ system but it has been reported there is a greater risk for toxicity with plasma levels greater than 10−5 M at 24 hours; greater than 5 × 10−7 M.4 MTX can lead to nephrotoxicity, myelosuppression, mucositis, hepatitis and dermatitis 5. Current clinical practice dictates that MTX be managed with vigorous hydration, urine alkalinization, therapeutic drug monitoring (TDM) and leucovorin (LV) rescue therapy. Empirically-developed nomograms (Figure 1) are often used (ideally, 24 to 36 hours post-infusion) to determine if patients are at high risk for MTX toxicity and to pharmacokinetically guide LV rescue therapy based on the TDM serum concentrations and the time post MTX infusion 2, 6, 7. All patients receiving MTX at the Children’s Hospital of Philadelphia are treated based on protocols supported by the Children’s Oncology Group. Likewise, these protocols outline specific MTX dosing and LV rescue requirements which vary in amount and duration of therapy as well as adjustment paradigms.
Figure 1.
Nomogram currently used by physicians to adjust leucovorin rescue therapy after receiving therapeutic drug monitoring data taken twenty-four hours after the start of methotrexate infusion (modified from Lexi-Comp).
Population pharmacokinetics and pharmacodynamics offer a mechanism to define sources of variation about the therapeutic window in target patient populations. Models derived from this approach do not have to remain static with respect to their ability to individualize new patient data as it becomes available. The prototype Bayesian forecasting algorithm described herein predicts MTX serum concentration in individual patients leveraging prior knowledge obtained from historical data and our current population pharmacokinetic model. The algorithm can be executed in real-time through incorporation into a broader decision support system interfaced with the Children’s Hospital of Philadelphia’s electronic medical record system8. This pediatric knowledgebase (PKB) seeks to inform caregivers and their patients with the state-of-the-art in dosing guidance in a dynamic manner that changes with patient status and in response to medical treatment. Specifically, the PKB system integrates static compendia drug information with drug-specific dashboards interfaced to the hospital-based medical records. Drug dashboards are designed for and by the physician therapeutic area in collaboration with clinical pharmacology and information technology expertise. The prototype methotrexate (MTX) dashboard described herein permits forecasting of plasma concentrations at select time points consistent with the specific clinical protocol used to manage the individual patient. The forecasting tool permits dosing scenarios to be explored via a user-friendly interface that front-ends a population-based PK/PD model. The PKB is a Java based, three-tier web application comprising a user interface (UI) tier, an application tier and a database tier. Initial testing and deployment has been limited to internet explorer, the official standard at CHOP. The objectives of this study were to (1) examine the predictive performance of the decision support aspects of the MTX dashboard regularly scheduled TDM observations based on within-patient, sequential data made available to the forecasting algorithm, and (2) to determine the start time and dosage of leucovorin rescue treatment based on these predictions to see if they concur with actual clinical practice. Our emphasis with this investigation was to develop and propose methodology by which such a system could be evaluated, not necessarily on the specific performance of the current version of the model / algorithm.
MATERIALS AND METHODS
DATA COLLECTION
The protocol for this investigation was approved by the Institutional review Board of the Children’s Hospital of Philadelphia. A waiver of HIPAA authorization under 45 CFR 165.512(i)(2)(ii) was granted based on the nature of the study evaluation. A waiver of assent and parental permission and consent was also granted because the study met the criteria under CFR 46.116(d), due to its de-identified and retrospective design. Medical records from fifty patients receiving methotrexate (mostly high-dose; > 1g/m2 in 48 of 50 patients) at the Children’s Hospital of Philadelphia were abstracted from the hospital’s EMR system. Of the total sampled population, 49 (of 50) patients received leucovorin (LV, folonic acid) rescue therapy along with their MTX infusion. While formal statistical analysis based on inference testing was not planned, the sample size of 50 was guided by the objective to detect a clinically-meaningful difference between predicted and observed concentrations at both end-infusion and C24 (concentration 24 hours post administration) for conventional MTX therapy.. The criterion was based on a two-sample t-test, alpha level of 5% and power requirement of 80%. Assumptions regarding variance and mean difference to be detected were based on the historical TDM data and input from oncology caregivers. At end-infusion, a standard deviation of 50 μM with a mean difference detected (observed vs. predicted) of 30μM was assumed while at C24 a standard deviation of 0.5μM and mean difference of 0.3μM was assumed. In both cases, a sample size of 50 subjects fulfilled the design requirements. All patients received HDMTX and had at least two observed MTX serum concentrations available in their medical records. Administrative and demographic data was obtained from source medical records (Chartmaxx and Sunrise Clinical Manager systems). MTX concentration collection times and the time that results were available were compared to determine the duration of time physicians must wait to receive information about their patients. Patients were chosen to have varied experience with MTX and demographic characteristics such as race, gender, age, height and weight. Some patients also received intrathecal doses of MTX during previous visits so the first cycle was considered to be the first time MTX was given when TDM was performed.
MTX STANDARD OF CARE AT CHILDREN’S HOSPITAL OF PHILADELPHIA
Before patients start HDMTX therapy, prehydration with 750 mL/m2 D5 0.22%NaCl and 40 mEq/L sodium bicarbonate (NaCHO3) is administered over one hour. If urine pH is less than 7, 0.5 mEq/kg of NaCHO3 is given over 30 minutes and hydration continues. This schedule is repeated if urine pH is less than 7 and HDMTX administration can only initiate once urine pH ≥ 7. Patients receive HDMTX as a 25 mg/ml solution in Dextrose 5% in water (D5W), with a maximum absolute dose of 20 g. Hydration is continued with D5 0.22% NaCl with 40 mEq/L NaHCO3 at 100 ml/m2/hour. If urine pH is less than 7, NaCHO3 is given and urine pH is checked again. TDM of MTX serum concentrations occurs 24 hours after infusion start and is performed every morning. Monitoring of urine pH, hydration and MTX serum levels continues until the MTX level ≤ 0.1 μM.
LV commences 24 to 42 hours (at the protocol-defined hour) after the start of HDMTX administration as an intravenous soluset (IVSS) over 15 minutes, every six hours until the MTX serum concentration is less than or 0.1 μM. The amount is protocol specific or if no protocol is assigned 10 mg/m2 is given for solid tumor treatment and 15 mg/m2 is given for leukemia/lymphoma treatment. TDM results are plotted on protocol-specific nomograms and the LV dose is adjusted if there is evidence of delayed MTX elimination or increased serum concentrations. Discharge is permitted only if the serum MTX level is 0.1 μM or less. Likewise, the actual serum MTX level varies across the patients evaluated in this analysis. All patients studied in this analysis were under the guidance of one of eight COG protocols in which MTX and LVR administration are advised. Hence, dosing was consistent with current standard of care and additional inclusion/exclusion criteria were not enforced.
MTX TDM ANALYSIS
Methotrexate serum concentrations were measured approximately every 24 hours after administration until they were less than or equal to 0.1 μM. The clinical pathology laboratory assays patient serum samples for methotrexate with the Abbott Diagnostics Inc. TDx system and Methotrexate II assay procedure using fluorescence polarization immunoassay (FPIA) detection in compliance with their procedure manuals and standard operating procedures. The laboratory runs this assay daily. The coefficient of variation for concentrations ranging from 0.07 to 500 μM was 14 to 5.5%. The assay has a sensitivity limit at less than 0.02 μM. Observations below this limit of quantification were omitted from the study.
METHOTREXATE MODEL
The underling population pharmacokinetic model used to characterize methotrexate disposition is based on a two-compartment disposition structural model with first-order elimination. Inter-subject variability is described with an exponential error model:
where, Pi is the estimated parameter value for individual i, P̂ is the typical population value (geometric mean) of the parameter, and ηPi are individual-specific interindividual random effects for individual i and parameter P and are assumed to be distributed: η ~ N(0,ω2) with covariance defined by the inter-individual covariance matrix Ω. The residual error was expressed by a proportional error model:
where, Cij is the jth measured observation in individual i, Ĉij is the jth model predicted value in individual i, and εij is the additive residual random error for individual i and measurement j and is assumed to be independently and identically distributed. Parameter values for the priors and their associated coefficients of variation (% CV) are found in Table 1. The model was developed from methotrexate dosing histories and monitored drug concentrations in 240 patients. The original dataset contained 2176 observations covering a range of one to 56 observations per patient (an average of 9 observations per patient). The age range was from 1 to 80 years with a weight range of 6.6 to 157 kg. The gender distribution was approximately 48% male (52% female). Hence, the underlying patient diversity permits consideration of relevant size and demographic dependencies though only the base model (without covariates) was utilized in this analysis. The residual error of 53.63% was relatively high but in line with previous MTX population models.
TABLE 1.
Population model parameter estimates used in the Bayesian forecasting algorithm
| Parameter | Estimate | Units | %CV |
|---|---|---|---|
| CLN | 7.49 | L/h | 6.37 |
| CLR | 2.55 | L/h | 75.46 |
| V1 | 36 | L | 19.03 |
| V2 | 3.33 | L | 52.15 |
| Q | .0984 | L/h | 12.25 |
CLN , clearance in patients with normal renal function; CLR, clearance in patients with impaired renal function; V1, volume of distribution in the central compartment; V2, volume of distribution in the peripheral compartment, Q, inter-compartmental clearance.
Unlike other MTX models recommended for individual patient-level prediction 9-14, our current MTX forecasting algorithm discriminates patients based on renal function (normal versus compromised renal function). Two clearance distributions are defined within the model as population priors (one for each renal function population based on mixture model on clearance from the original 240 patient dataset). Based on individual patient MTX serum concentrations, a patient is assigned to one of the two populations. Bayesian prediction of MTX concentrations is performed with the NONMEM prior subroutine, which incorporates the population model parameter estimates into the forecasting model. The Bayesian objective function (−2log(L(θ)) accounts for both the patient (individual) data and the population values:
where, L is the likelihood, θ is the parameter, σ is the within-individual variability, and ω is the inter-individual variability. The first term on the right side of the equation is driven by the individual’s data and the second term is driven by the historical population. When ω << σ, the estimate is influenced more by the patient data and when σ << ω the estimate is influence more by the prior.
PREDICITIVE PERFORMANCE
The predictive performance of the Bayesian MTX forecasting algorithm was evaluated based on 50 patients from the Children’s Hospital of Philadelphia’s historical electronic medical records. For each patient, the model was run sequentially in the NONMEM software version V (ICON, Ellicott City, MD) at each time the patient had a measured MTX concentration as part of normal TDM procedures. For the first observation, the population model parameter estimates were solely used to guide the prediction. For each subsequent observation, the MTX algorithm was run sequentially including the “new” observation to that patient’s dataset, incorporating a new set of model priors obtained from the previous run, and predicting the next MTX serum concentration. This was repeated for up to five observations where actual measured MTX concentrations occurred. If a patient did not have five TDM observations, this was repeated for all available TDM observations.
A SAS script was written to execute NONMEM and update the control files with the new prior information for all fifty patients. A runlog script within the NONMEM control file produces a comma-separated values file that contains the values of the NONMEM generated parameters. The SAS script imports the control and runlog files. It then reads the values of the model parameters (thetas and etas) from this runlog and updates the control file.
STATISTICAL ANALYSIS
The individual predicted concentrations generated by the forecasting algorithm were compared to the actual observed concentrations obtained from the patient medical records for all of the time points. The difference between these values, the prediction error (pe), was determined and the precision and the bias of the forecasting algorithm were assessed via the root mean squared prediction error (rmse) and the mean prediction error (me), respectively,15 as defined below:
A one-way, nested (run within subject) analysis of variance (ANOVA) was performed to determine if there was a difference between the means of the prediction error for each run differ. A pairwise procedure (Tukey’s) was then performed to determine the difference between subsequent runs. All statistical analyses were performed with SAS version 9.1 for PC/Windows. Statistical significance was considered at the p < 0.05 level.
RESCUE THERAPY GUIDANCE
The concordance between rescue therapy guidance predicted from the MTX DSS and the actual LV administration captured in the patient’s medical records was also evaluated. Specifically, predicted MTX exposures were overlaid on the LV nomogram and the actual clinical outcome (e.g., whether and/or when leucovorin dose or rescue actually occurred, dose amount) was compared with the intersection of the model predicted exposure and the LV nomogram with respect to LV dose and timing. The number of times that LV was actually and predicted to be administered at the same time, the number of times that it was predicted that leucovorin dosing to occur prior to actual dosing and the number of times that the actual dosing occurred before the predicted were all determined.
RESULTS
Forecasting MTX Exposure
The test population consisted of 50 patients, 29 male (21 female), visiting the Children’s Hospital of Philadelphia from November 2004 to December 2009 with age ranging from 6 months to 21 years . Table 2 provides a summary of the patient demographics and observed TDM response over time relative to dosing event. There were 183 TDM observations predicted ranging from 0.1 μM to 184.85 μM. The time to reach the threshold MTX concentration took as little as 36 hours to over 5 days. It took 72 hours for 60% (30 patients) of the patients to reach the threshold MTX concentration with it taking 96 hours for 60% (12 patients) of the remaining to reach the threshold. LV commenced at 36 hours for 36% (18 patients) of the patients and at 42 hours for 81% (26 patients) of the remaining patients.
TABLE 2.
Summary of test patient characteristics
| N | Units | Mean ± Standard Deviation | Range | |
|---|---|---|---|---|
| Weight | 50 | kg | 45.2 ± 30.1 | 7.6 – 163.3 |
| Age | 50 | years | 11.0 ± 5.7 | 0.5 – 21.0 |
| Body surface area | 50 | m2 | 1.3 ± 0.5 | 0.4 – 2.4 |
| MTX Dose | 80 | mg | 6607 ± 4912 | 140 – 20000 |
| MTX Dose | 80 | mg/m2 | 4885 ± 2605 | 347 – 12652 |
| First observation | 50 | (μM) | 20.60 ± 32.13 | 0.34 – 184.85 |
| Second observation | 50 | (μM) | 1.34 ± 2.33 | 0.05 – 15.32 |
| Third observation | 43 | (μM) | 0.21 ± 0.33 | 0.01 – 1.99 |
| Fourth observation | 28 | (μM) | 0.17 ± 0.23 | 0.02 – 0.85 |
| Fifth observation | 12 | (μM) | 0.16 ± 0.14 | 0.04 – 0.42 |
Observation refers to the measured MTX serum concentration
Figure 2 shows that as the run number increases, the prediction error and therefore the bias both decrease. The precision (root mean squared error) was found to be 12.85% and the overall bias (mean error) was 2.15%. This is expected as the prediction becomes more informed by the individual patient data as opposed to the priors. The magnitude of the precision error is reduced as the run number increases. This relationship was not statistically significant, however. Analysis of variance showed that the effect of run number on the prediction error had a p-value of 0.3206 and the number of total concentrations (for a given patient) did not significantly affect the prediction error (p = 0.5375). Furthermore, pair-wise comparison of means showed that after the first run the predictive power of the algorithm remained the same after one observation was available to the forecasting algorithm. There was no significance found between any of the runs.
Figure 2.
Effect of the run number and the number of observations on the precision error of the model in forecasting methotrexate serum concentrations. As the number of runs increases, the precision error decreases.
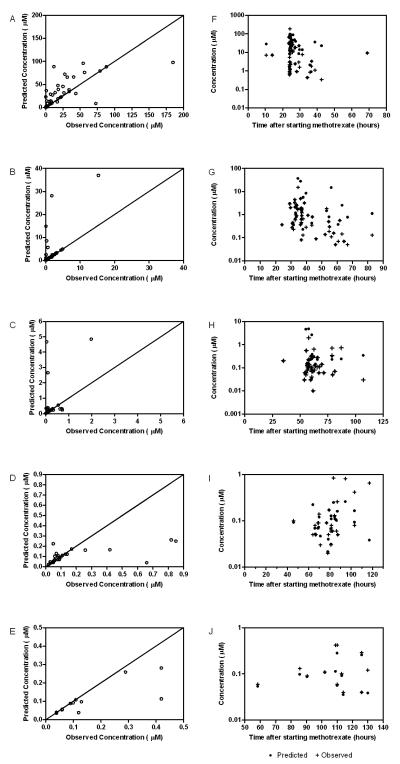
Diagnostic plots of predicted concentration versus observed concentration for each run are shown in Figure 3. Subject 31 appears to be a significant outlier in all of these plots. Although there seems to be poor prediction ability, this can be explained, in part, by the high inter-individual and residual variability of the forecasting algorithm. The model has been previously validated with more densely-sampled data while this application and analysis is strictly applied to the TDM / clinical setting. This patient is examined more critically below.
Figure 3.
Individual predicted concentration from the Bayesian forecasting algorithm versus the observed TDM concentrations. Predictions were made with one (A), two (B), three (C), four (D), or five (E) observations.
LV Rescue Guidance
The first MTX serum concentration was not observed until after 42 hours post infusion for 19 of the 50 patients. Twenty-nine patients started LV rescue before their MTX serum concentrations were received by the physician, therefore the dose of LV was not based on the individual’s serum concentration along with a nomogram was not used. Since serum samples are collected and the results are received at a later time there is a lag time that caregivers receive patient information. From the 50 patients in the validation population, the time between collection and result averaged to be 6.95 hours, with it taking as little as 40 minutes but as long as 29 hours to receive the result. Furthermore, the first observation for each of the 50 patients was received an average of 40 hours after the start of the MTX infusion, with it taking 36 hours or longer for 40 (80%) of the patient’s result to be available to the caregiver.
Patient predictions were overlaid with the LV nomogram utilized in the Children’s Hospital of Philadelphia’s formulary. Based on this review, 16 patients would have commenced LV earlier than when they actually started, 33 would have received it later and one did not need LV rescue. 7 patients would have received a larger dose of LV and 42 would have received less. Finally, for 37 of the patients, LV would have been administered less often.
Patient-specific Assessment
For certain patients the algorithm performed extremely well or was consistently off. The HDMTX experience for two patients follows:
Case Study 1: Subject 30 (Figure 4A)
Figure 4.
Leucovorin guidance nomogram overlaid with TDM and predicted data for (A) subject 30 (good fit) and (B) subject 31 (poor fit).
The patient is a 14 year old, white female (46.8 kg, 1.42 m2) diagnosed with osteosarcoma, on her first cycle of HDMTX. She received 18000 mg of MTX over four hours. The first TDM sample was drawn at 24.5 hours after MTX commenced, was received at 42.48 hours and was 17.95 μM. LV began at 24.5 hours as well and was 16 mg/m2 every 6 hours. The LV dose did not have to be adjusted and she was discharged the same day that the threshold concentration of 0.08 μM was reached 81.3 hours after MTX commenced. The final result was received 5 hours after it was drawn.
Case Study 2: Subject 31 (Figure 4B)
The patient was a 12 year old, white male (47.7 kg, 1.45 m2) diagnosed with ALL on his first cycle of HDMTX. He received a 725 mg bolus IV of MTX over 30 minutes, followed by a 6600 mg infusion over another 23.5 hours. His first MTX sample was taken one half hour after the MTX infusion finished and was 184.85 μM. LV rescue commenced at approximately 42 hours and was 15 mg/m2 every six hours. The threshold MTX concentration was not reached until the sample taken at 226 hours which was not received by the caregiver until 8.5 hours later. He was discharged that day. At 66.5 hours the frequency of LV administration was increased to every three hours after a concentration taken at 60 hours yielded an MTX serum concentration of 2μM.
DISCUSSION
Currently, MTX is the only antineoplastic agent routinely monitored in the pediatric oncology setting 16, with TDM observations being used solely to determine whether the dose of LV should be increased due to delayed excretion. The need for individualized LV rescue is apparent because of the high variability of MTX response between patients and its narrow therapeutic window 2, 4. If patients receive too much LV, MTX can become inefficacious and the possibility of relapse increases17, 18. In contrast, if too little LV is given it can lead to the MTX associated toxicities. Although LV administration is based on the individual’s clearance of MTX, there are still reports of toxicity and death in patients receiving HDMTX 19-21. These studies imply that earlier commencement and an increase in the amount of LV given could have prevented these toxicities and fatalities. Nomograms are used to adjust the LV dose and hydration rate if there is evidence of delayed MTX elimination and/or renal toxicity. Based on the first serum concentration and nomogram, twelve of the patients would have received a different dose of LV. Since it took longer than 36 hours for the majority of the patient’s first MTX concentration to come back, the patient could have been identified for potential toxicity sooner and rescue therapy could have been considered in light of the data. Also, the practice of collecting TDM samples every 24 hours delays the time for the caregiver to be informed about the patient. This can lead to patients remaining in the hospital long after their MTX serum concentration has fallen below the threshold concentration to be discharged 22, leading to poor bed management and increased costs for the patient and their families.
MTX is well managed by caregivers with aggressive hydration, TDM of serum concentrations and administration of LV rescue therapy. However, current MTX practice is often guided by population means in the form of nomograms. This practice is essentially empirical, not based on the individual and is static with respect to evolving learned guidance from future visits or patients. Our emphasis with the dashboard approach is to inform the caregiver based on clinically-relevant indices of patient well-being and the response to MTX therapy (see Figure 5 for MTX dashboard screen shot illustrating multi-view panels of recent dosing event)
Figure 5.
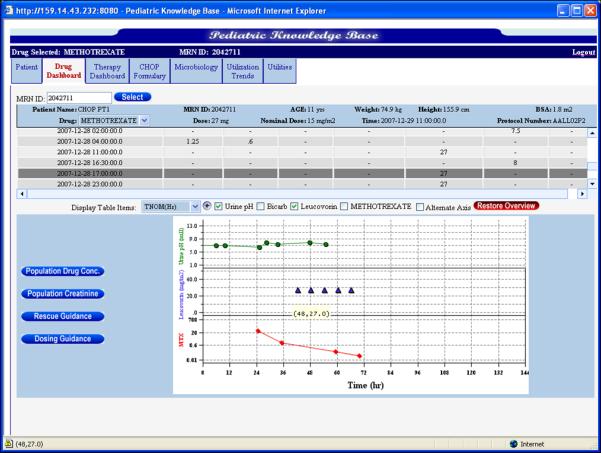
MTX dashboard screen shot illustrating patient-specific views to multiple indices of patient response to dosing.
Although the pharmacokinetics of MTX have been modeled in both adult and pediatric populations 11, 13, 14, 23-27 they are solely used in the realm of research and have not been applied to the clinical setting in order to provide guidance to caregivers. Because of the threat of toxicity, the ability for the caregiver to have an idea of the MTX serum concentration immediately after MTX initiation using the knowledge from the historical population and dose amount would become a valuable resource to have early detection of delayed excretion and commence rescue treatments sooner. While most of the clinical concern surrounds the idiosyncratic toxicities of MTX therapy, the incorporation of the MTX forecasting algorithm in the clinical setting would provide the caregiver with more information about the individual patient’s concentration-time profile which would optimize LV rescue therapy and likely predict poor renal impairment outcomes (nephrotoxicity, mucositis, etc.).
This retrospective study evaluates the clinical performance of a Bayesian population pharmacokinetic model developed using the NONMEM software in the pediatric oncology setting using TDM data. An important challenge for this system is the uncertainty in the collection and reporting of TDM levels as well as the accuracy of the dosing records. The Bayesian estimator incorporates both the individual’s data and the data from the historical population used to develop the model though future versions of this solution will address the stochastic nature of the dosing records as well as the prediction engine (i.e., other than NONMEM). At the moment, measurement error associated with the analytical method is lumped in with the residual error in the population model. Future model representations will separate these contributions in a more discriminate matter and it may well be possible to improve prediction by reducing assay variability via refined analytical methods. In any case, it is apparent that after the second observation that the estimate becomes patient data driven with less influence by the population model parameters. While this is expected given the nature of the Bayesian objective function, future models will accommodate more patient-specific factors, such as weight, BSA, and leucovorin dose, relevant to the management of HDMTX and are expected to further improve prediction accuracy.
Since this preliminary model was built with both pediatric and adult patients, initially the priors had to be updated to better account for the solely pediatric and sparsely sampled via TDM population at the Children’s Hospital of Philadelphia. There will be little need to update priors in the final production version of the MTX DSS given that these will reflect a broad population at the Children’s Hospital of Philadelphia once additional data is incorporated and hence informative priors. Future model development will include and extensive covariate analysis beyond a simple adjustment for renal function. Early results seem very promising in this regard. In addition, a follow-up, prospective standard of care study is being planned to examine the relationship between MTX, LVR and folinic acid levels with consideration for genetic polymorphisms that may also explain some of the variability in MTX pharmacokinetics. The current version of the MTX dashboard will be evaluated as well in this study so that useability from the caregiver’s perspective can be evaluated. Finally, other forecasting solutions will be explored to determine if improvements in prediction accuracy can be made. In this regard, our current results serve as a baseline with respect to the evaluation of the MTX dashboard as it evolves.
CONCLUSIONS
We have described a process by which a MTX decision support system can be evaluated retrospectively for clinical utility in managing high-dose MTX pharmacotherapy in children with cancer. Given the empirical and somewhat static nature of the current practice, the MTX dashboard will provide caregivers with more informative MTX guidance as TDM data is collected. Based on a single TDM observation and a model which quantitatively partitions variability between patients and adjusts for renal function, prediction of future TDM observations is possible.
The forecasting algorithm will continue to evolve and is the subject of an active research program in our group. The evaluation of patient-specific covariates such as body surface area, weight, age, race, serum creatinine, creatinine clearance, LV dose and hydration dose is ongoing to further address the high inter-individual variability and residual error. In addition, recent studies have shown a correlation between MTX clearance and various SNPs. With the available genomic data at the Children’s Hospital of Philadelphia, these SNPs will be investigated as potential covariates in the forecasting algorithm as well.
Prior to production, the dashboard will be integrated with the EMR systems used at the Children’s Hospital of Philadelphia, Sunrise Clinical Manager (http://eclipsnet.com/solutions/clinical_solutions.asp?id=1) and Epic (http://www.epic.com/). Considerations for other EMR systems are intended and the production code will likely not require the NONMEM algorithm. The vision for the pediatric knowledgebase and the individual drug dashboards specifically involves periodic re-evaluation of the models, predictive capabilities and performance against the currently available clinical and diagnostic data from which they are constructed. Likewise, procedures such as those described herein will need to be challenged and replicated as these systems evolve.
ACKNOWLEDGEMENTS
We are very grateful to Dr. John Mondick, previously a member of the Laboratory for Applied PK/PD and now with the Metrum Research Institute, for his efforts on the development of the initial methotrexate population pharmacokinetic model.
FUNDING SUPPORT: This work was partially supported by NIH/NICHD, Pediatric Pharmacology Research Unit, Grant # HD037255-06, NICHD/NLM, Grant # 1RC1LM010367-01, Decision Support System to Guide Pediatric Pharmacotherapy and an internal grant from the Pediatric’s Chair’s Initiative of the Children’s Hospital of Philadelphia
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Barrett JS, Patel D, Jayaraman B, et al. Key Performance Indicators for the Assessment of Pediatric Pharmacotherapeutic Guidance. Journal of Pediatric Pharmacology and Therapeutics. 2008;13(3):141–155. doi: 10.5863/1551-6776-13.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11(6):694–703. doi: 10.1634/theoncologist.11-6-694. [DOI] [PubMed] [Google Scholar]
- 3.Bleyer WA. Methotrexate: clinical pharmacology, current status and therapeutic guidelines. Cancer Treat Rev. 1977;4(2):87–101. doi: 10.1016/s0305-7372(77)80007-8. [DOI] [PubMed] [Google Scholar]
- 4.Evans WE, Pratt CB, Taylor RH, et al. Pharmacokinetic monitoring of high-dose methotrexate. Early recognition of high-risk patients. Cancer Chemother Pharmacol. 1979;3(3):161–6. doi: 10.1007/BF00262416. [DOI] [PubMed] [Google Scholar]
- 5.Widemann BC, Balis FM, Kempf-Bielack B, et al. High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma. Cancer. 2004;100(10):2222–32. doi: 10.1002/cncr.20255. [DOI] [PubMed] [Google Scholar]
- 6.Treon SP, Chabner BA. Concepts in use of high-dose methotrexate therapy. Clin Chem. 1996;42(8 Pt 2):1322–9. [PubMed] [Google Scholar]
- 7.Bleyer WA. The clinical pharmacology of methotrexate: new applications of an old drug. Cancer. 1978;41(1):36–51. doi: 10.1002/1097-0142(197801)41:1<36::aid-cncr2820410108>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 8.Barrett JS, Mondick JT, Narayan M, et al. Integration of modeling and simulation into hospital-based decision support systems guiding pediatric pharmacotherapy. BMC Med Inform Decis Mak. 2008;8:6. doi: 10.1186/1472-6947-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruno R, Iliadis A, Favre R, et al. Dosage predictions in high-dose methotrexate infusions. Part 2: Bayesian estimation of methotrexate clearance. Cancer Drug Deliv. 1985;2(4):277–83. doi: 10.1089/cdd.1985.2.277. [DOI] [PubMed] [Google Scholar]
- 10.Monjanel-Mouterde S, Lejeune C, Ciccolini J, et al. Bayesian population model of methotrexate to guide dosage adjustments for folate rescue in patients with breast cancer. J Clin Pharm Ther. 2002;27(3):189–95. doi: 10.1046/j.1365-2710.2002.00402.x. [DOI] [PubMed] [Google Scholar]
- 11.Odoul F, Le Guellec C, Lamagnere JP, et al. Prediction of methotrexate elimination after high dose infusion in children with acute lymphoblastic leukaemia using a population pharmacokinetic approach. Fundam Clin Pharmacol. 1999;13(5):595–604. doi: 10.1111/j.1472-8206.1999.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 12.Pignon T, Lacarelle B, Duffaud F, et al. Pharmacokinetics of high-dose methotrexate in adult osteogenic sarcoma. Cancer Chemother Pharmacol. 1994;33(5):420–4. doi: 10.1007/BF00686272. [DOI] [PubMed] [Google Scholar]
- 13.Plard C, Bressolle F, Fakhoury M, et al. A limited sampling strategy to estimate individual pharmacokinetic parameters of methotrexate in children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2007;60(4):609–20. doi: 10.1007/s00280-006-0394-3. [DOI] [PubMed] [Google Scholar]
- 14.Rousseau A, Sabot C, Delepine N, et al. Bayesian estimation of methotrexate pharmacokinetic parameters and area under the curve in children and young adults with localised osteosarcoma. Clin Pharmacokinet. 2002;41(13):1095–104. doi: 10.2165/00003088-200241130-00006. [DOI] [PubMed] [Google Scholar]
- 15.Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9(4):503–12. doi: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- 16.Hon YY, Evans WE. Making TDM work to optimize cancer chemotherapy: a multidisciplinary team approach. Clin Chem. 1998;44(2):388–400. [PubMed] [Google Scholar]
- 17.Fotoohi K, Skarby T, Soderhall S, et al. Interference of 7-hydroxymethotrexate with the determination of methotrexate in plasma samples from children with acute lymphoblastic leukemia employing routine clinical assays. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;817(2):139–44. doi: 10.1016/j.jchromb.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 18.Relling MV, Fairclough D, Ayers D, et al. Patient characteristics associated with high-risk methotrexate concentrations and toxicity. J Clin Oncol. 1994;12(8):1667–72. doi: 10.1200/JCO.1994.12.8.1667. [DOI] [PubMed] [Google Scholar]
- 19.Haim N, Kedar A, Robinson E. Methotrexate-related deaths in patients previously treated with cis-diamminedichloride platinum. Cancer Chemother Pharmacol. 1984;13(3):223–5. doi: 10.1007/BF00269034. [DOI] [PubMed] [Google Scholar]
- 20.Jurgens H, Beron G, Winkler K. Toxicity associated with combination chemotherapy for osteosarcoma: a report of the cooperative osteosarcoma study (COSS 80) J Cancer Res Clin Oncol. 1983;106(Suppl):14–8. doi: 10.1007/BF00625045. [DOI] [PubMed] [Google Scholar]
- 21.Stark AN, Jackson G, Carey PJ, et al. Severe renal toxicity due to intermediate-dose methotrexate. Cancer Chemother Pharmacol. 1989;24(4):243–5. doi: 10.1007/BF00257626. [DOI] [PubMed] [Google Scholar]
- 22.Faltaos DW, Hulot JS, Urien S, et al. Population pharmacokinetic study of methotrexate in patients with lymphoid malignancy. Cancer Chemother Pharmacol. 2006;58(5):626–33. doi: 10.1007/s00280-006-0202-0. [DOI] [PubMed] [Google Scholar]
- 23.Aquerreta I, Aldaz A, Giraldez J, et al. Pharmacodynamics of high-dose methotrexate in pediatric patients. Ann Pharmacother. 2002;36(9):1344–50. doi: 10.1345/aph.1A446. [DOI] [PubMed] [Google Scholar]
- 24.Aumente D, Buelga DS, Lukas JC, et al. Population pharmacokinetics of high-dose methotrexate in children with acute lymphoblastic leukaemia. Clin Pharmacokinet. 2006;45(12):1227–38. doi: 10.2165/00003088-200645120-00007. [DOI] [PubMed] [Google Scholar]
- 25.Evans WE, Crom WR, Abromowitch M, et al. Clinical pharmacodynamics of high-dose methotrexate in acute lymphocytic leukemia. Identification of a relation between concentration and effect. N Engl J Med. 1986;314(8):471–7. doi: 10.1056/NEJM198602203140803. [DOI] [PubMed] [Google Scholar]
- 26.Evans WE, Crom WR, Stewart CF, et al. Methotrexate systemic clearance influences probability of relapse in children with standard-risk acute lymphocytic leukaemia. Lancet. 1984;1(8373):359–62. doi: 10.1016/s0140-6736(84)90411-2. [DOI] [PubMed] [Google Scholar]
- 27.Thompson PA, Murry DJ, Rosner GL, et al. Methotrexate pharmacokinetics in infants with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2007;59(6):847–53. doi: 10.1007/s00280-006-0388-1. [DOI] [PubMed] [Google Scholar]