Fig. 1.
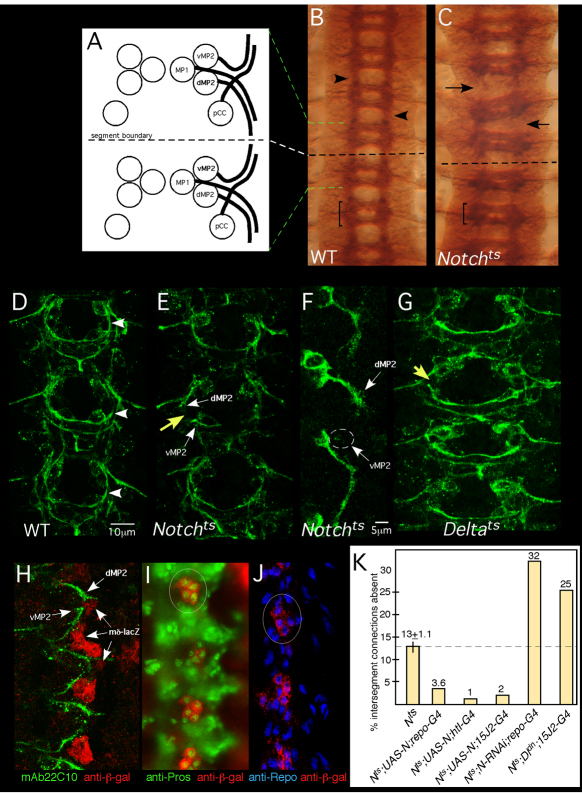
Notch is required for growth and guidance of longitudinal pioneers. (A) Four pioneer neurons establish intersegmental connections in the Drosophila CNS: pCC, MP1, dMP2 and vMP2. Two neuromeres and the intersegmental region between are shown. Green and white dashed lines indicate alignment of the schematic to the micrograph in B. Anterior is to the top. (B,C) Axon patterning in the mature embryonic nerve cord. Wild-type (WT; B) or Notchts (C) embryos were shifted to 32°C before pioneer axons extend, grown to stage 15/16, fixed and stained with mAb BP102. Arrows in C indicate gaps between adjacent segments in the mutant; compare with wild type (arrowheads in B). Bracket indicates a single neuromere. (D,E,G) Axon patterning in early CNS. WT (D), Notchts (E) or Deltats (G) embryos were shifted to 32°C then fixed at mid-stage 13 and stained with mAb 22C10 to reveal pioneer axons. Yellow arrows indicate missing longitudinal connections in the mutants; compare with wild type (arrowhead in D). White arrows highlight stalled dMP2 and vMP2 growth cones in Notchts. (F) dMP2 and vMP2 neurons in Notchts. A mid-stage 13 Notchts embryo expressing mCD8-GFP in dMP2 and vMP2 under control of GAL4-15J2 was prepared as described for D,E and visualized with anti-GFP. Arrows indicate stalled MP2 growth cones. Wisps of projections from a stalled vMP2 growth cone are encircled. (H) Wild-type early stage 13 embryo bearing a lacZ reporter for Notch signaling activity [E(spl)mδ-lacZ], labeled with anti-β-galactosidase (β-gal; red) and mAb22C10 (green). Arrows highlight the proximity of pioneer growth cones to cells with activated Notch signaling (lacZ+). (I,J) Stage 13 embryos bearing the E(spl)mδ-lacZ reporter, labeled with anti-β-gal (red) and markers for interface glia, anti-Prospero (I, green) or anti-Repo (J, blue). Circles highlight clusters of β-gal-positive cells. (K) Notchts embryos bearing the indicated transgenes were prepared as described for D,E and intersegmental connections that failed to form by mid-late stage 13 were quantified. All deviations from Notchts are statistically significant (P<0.05; ANOVA). The thin vertical line on the Notch bar shows s.e.m. None of the transgenes produced dominant defects in a wild-type background under these conditions (<2% of hemisegments).