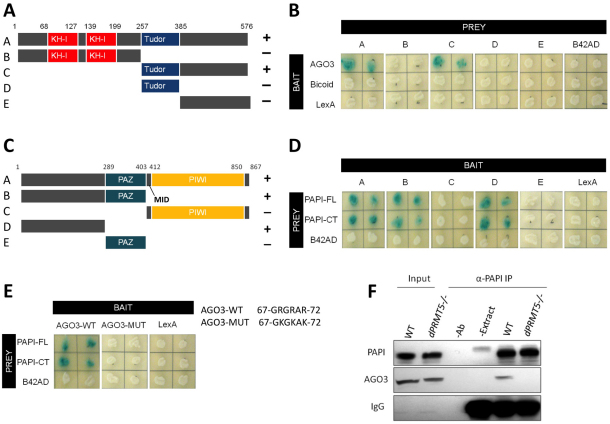
Fig. 2.
PAPI binds to the sDMAs in the N terminus of AGO3 via its TUDOR domain. (A) PAPI deletions used to map the AGO3-binding region. The positions of amino acid residues at various domain junctions are indicated. (B) Y2H assay indicating that the C-terminal domain of PAPI interacts with AGO3. Drosophila Bicoid protein and LexA were used as negative controls. (C) AGO3 deletions used to map the PAPI-binding region. The positions of amino acid residues at various domain junctions are indicated. (D) Y2H assay indicating that the N-terminal domain of AGO3 interacts with PAPI-FL and PAPI-CT. The B42AD moiety of the prey plasmid pJG4-5 was used as a negative control. (E) The sDMAs in the N terminus of AGO3 are required for the PAPI-AGO3 interaction. Triple sDMA mutant AGO3 (AGO3-MUT) does not interact with PAPI. Arginine to lysine mutations are indicated. (F) PAPI immunoprecipitates from wild-type (WT) and dPRMT5 mutant ovaries were subjected to western blotting analysis and probed with anti-PAPI and anti-AGO3 antibodies. AGO3 is detected only in the PAPI immunoprecipitates from wild-type ovaries. Extract plus beads (−Ab) and antibody plus beads (−Extract) were used as negative controls for IP. IgG was probed as a loading control.