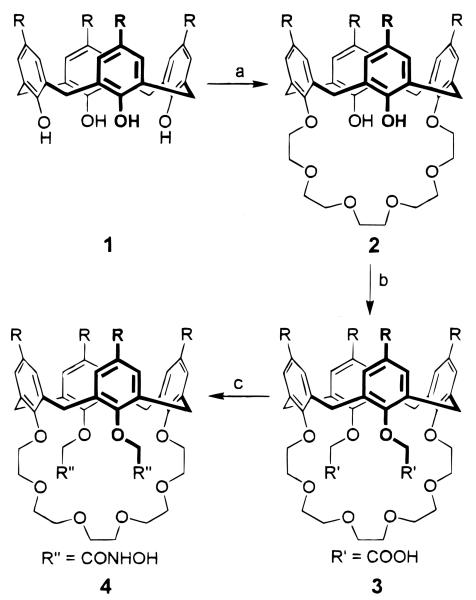
Figure 1.
Preparation of the proton-ionizable calixarene-crown ethers p-tert-butylcalix[4]arene-crown-6-dicarboxylic acid (3) and p-tert-butylcalix[4]arene-crown-6-dihydroxamic acid (4). Reagents: (a) Ts(OCH2CH2)5OTs, Cs2CO3, CH3CN, reflux 24 h, 32%; (b) (i) NaH, BrCH2COOEt, THF, reflux 5 h, 73%; (ii) Et4NOH, THF–H2O, reflux 24 h, 82%; (c) (i) (COCl)2; (ii) O-benzylhydroxamine, pyridine, 35 °C, 24 h, 65%; (iii) H2/Pd–C, HOAc–MeOH, 92%.