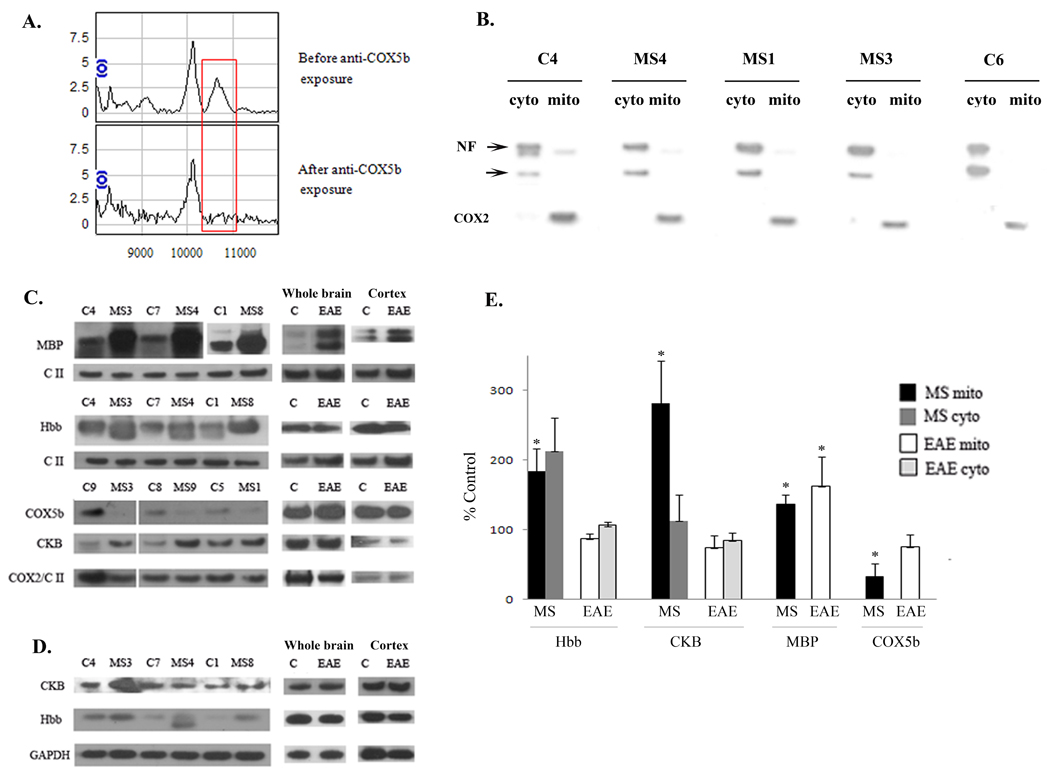
Figure 3. Confirmation of the identity of differentially expressed proteins.
A. Mitochondrially enriched protein solutions were incubated with an antibody to COX5b covalently bound to beads. Aliquots analyzed by SELDI-TOF-MS before and after incubation confirm the identity of the mass spectral peak at 10.6 kDa as COX5b. The red box highlights the region of interest for these spectra. B. Representative western blot demonstrating the relative purity of the cellular fractionation. Western blots were performed on cytoplasmic (cyto) and mitochondrial (mito) fractions isolated from MS and control cortex, run side by side and blotted with an antibody to the neuron specific protein, neurofilament (NF), and an antibody to the mitochondrial encoded COX2 protein. Multiple NF immunoreactive proteins are denoted by arrows. C. Representative western blots show increased MBP, hemoglobin β (Hbb), and creatine kinase (CKB) and decreased COX5b in mitochondrial fractions isolated from MS cortex. Increased expression of MBP was also observed in the EAE mouse brain while CKB, Hbb, and COX5b were not similarly altered in EAE and MS. To control for protein loading, western blots were reprobed with antibodies to either Complex II (CII) or COX2. D. Representative western blots of hemoglobin β (Hbb) and creatine kinase B (CKB) in cytoplasmic fractions isolated from MS and control cortex and also from EAE and control brains or cortex with GAPDH as a loading control. E. Quantitation was done for MBP, COX5b, Hbb, and CKB expression for MS and control samples and for EAE cortex. Data is expressed as percent of control for MS and EAE samples and densitometry was standardized to either CII or COX2 for mitochondrial fractions or to GAPDH for cytoplasmic fractions. Error bars represent SEM. * p≤ 0.05