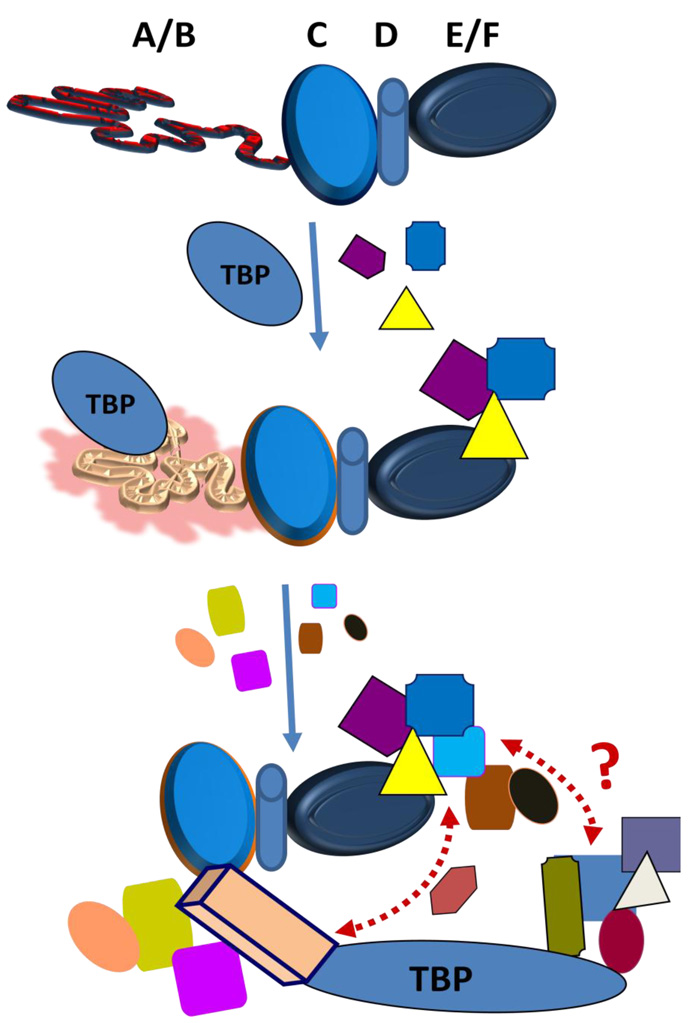
Figure 4. A hypothetical model of folding of the SRs’ AF1 domain in the context of full length receptor under physiological conditions.
The ID AF1 domain interacts with one or more proteins from the basal transcription initiation machinery complex, e.g. TBP and in doing so acquires a set of conformations that allows AF1 surfaces to create a platform for interaction with other coregulatory proteins to carry out its function. The resulting structurally modified forms of AF1 may activate it for interactions with various other critical coregulatory proteins essential for gene regulation by the receptor. These interactions give the final folded structure to AF1 and form the basis for the multi-protein assemblies involved in SR-mediated regulation of transcription. This induced fit model of AF1 folding infers that AF1 is not fully structured in vivo until it binds one or another key partner molecule (shown by different colors and shapes). This induced-conformation or limited set of conformations in AF1 is needed in order for it to carry out its transcription function. Formally, induced fit could occur by initial non-specific interactions between the unfolded AF1 domain and the BP. In this version of the model, when such interactions occur, the proximity of the two proteins leads to rapid acquisition of the proper structure in AF1. Alternatively, there initially could be more specific interactions of coregulatory proteins with a partially folded AF1, or even with a tiny pool of fully folded AF1 molecules, creating a kinetic “sink” into which the general population falls.