Figure 5.
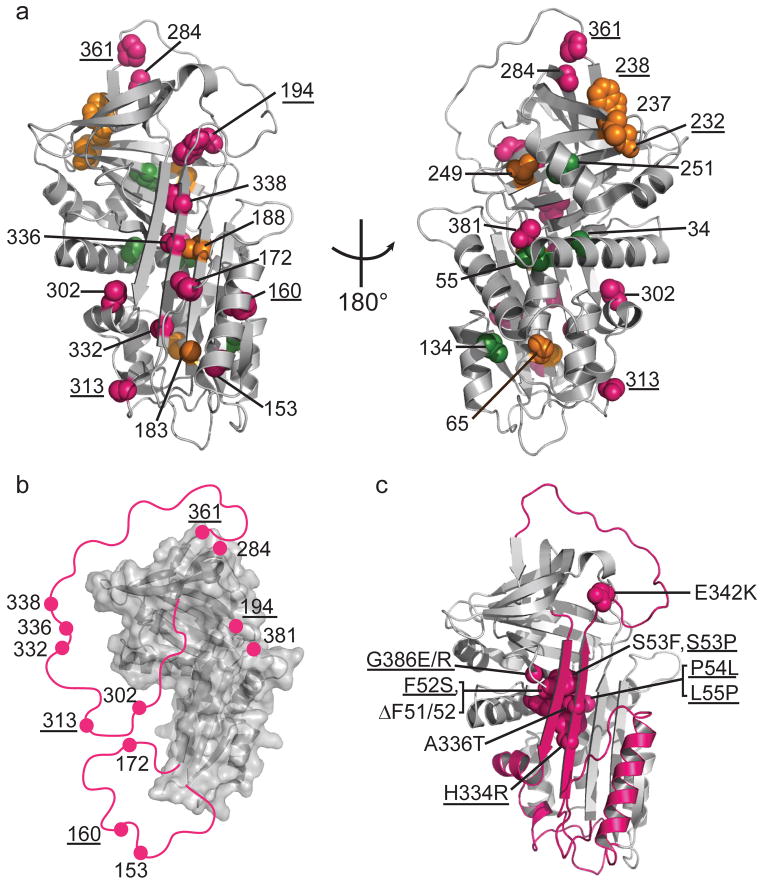
Local unfolding leads to the sheet-opened intermediate of α1AT. (a) Cysteine positions are shown on the structure of native α1AT (PDB 1QLP)31 and colored by their accessibility behavior with pink, green and orange representing Class I, Class II, and III, respectively (see Fig. 2 and text for description of classes). Results for an additional six residue positions from previous work12,13,19 are indicated with appropriate coloring on the structure as well (labels are underlined) using the same color scheme. (b) The stable structural elements of the sheet-opened intermediate as inferred from cysteine accessibility data. In this structure, regions found to be accessible in the sheet-opened intermediate of α1AT are removed and indicated schematically by a pink line connecting to the remaining structure. (c) The five naturally occurring pathogenic point mutations in α1AT that produce full-length, polymerogenic protein and cause liver damage25,27 along with similar polymerogenic mutations reported for other serpins7 (underlined labels) are represented in pink spacefill on the native structure of α1AT. The backbone of the identified labile region is in pink.