Figure 1.
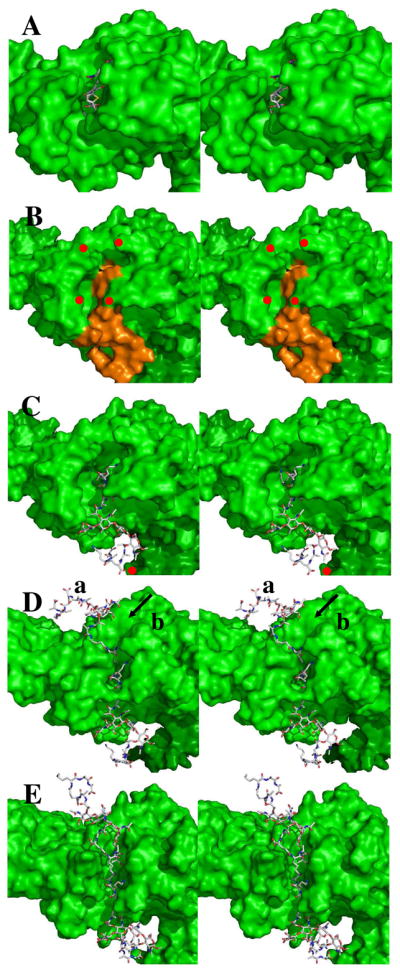
Panel A shows the stereo structure (2UWX) of acylated PBP 1b (depicted as a Connolley surface) with the nitrocefin-derived acyl-enzyme (shown in capped-stick: C, gray; N, blue; O, red; H, cyan; S, yellow). Panel B shows the docked Michaelis complex of the NAG-NAM-NAG-NAM substrate (orange) in the PBP 1b structure (green surface, showing red dots to identify residues Asn500 as the top left red dot; Gln686, top right; Asn494, bottom left; and Asn656, bottom right); Panel C is identical to Panel B with the exception that the docked substrate is in capped-stick representation. The angle of the pentapeptide stems relative to the glycan strand is 110° (± 10°). Panel D shows the probable location (mode a) of the acceptor stem engagement of the acyl-enzyme and an alternative (and less probable) mode b cleft for acceptor stem approach (arrowed). Panel E shows the opened cleft, derived from 10 ns MD simulation of the structure of Panel D. In Panel E the separation between the δNH2 of Asn494 and the δNH2 of Asn656 is 5 Å. The plane of the bacterial membrane to which this enzyme is embedded is located distant to the right edge of these panels.
