Fig 2.
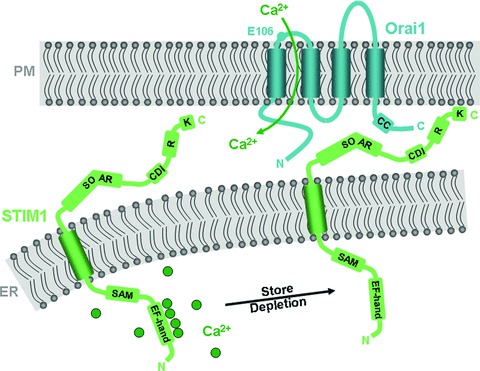
Predicted topologies and functional domains of STIM1 and Orai1 proteins. Shown for STIM1 are the Ca2+-sensing EF-hand domain and the SAM in the ER lumen, and the SOAR, the Ca2+-dependent inactivation (CDI) domain, the regulatory domain that is modified by phosphorylation (R) and the poly-lysine (K) domain in the cytoplasm. As illustrated, STIM1 rearranges within the ER membrane in response to Ca2+ store depletion, allowing it to interact with and activate Orai1 channels in the plasma membrane. Orai1 is predicted to have four transmembrane regions, such that its N- and C-termini are cytoplasmic. Shown are E106, an amino acid within the channel pore that confers Ca2+ selectivity, and a putative coiled-coil in the C-terminus that is required for interaction with and activation by STIM1.
