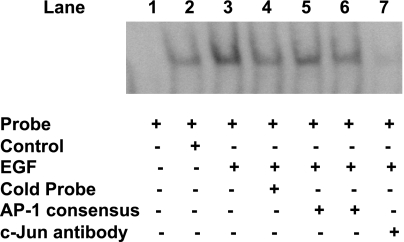
Fig. 7.
c-jun binds to potential AP-1 cis-elements of hSERTp2. Nuclear extracts from control (untreated) or EGF-treated (10 ng/ml, 24 h) Caco-2 cells were incubated with DIG-labeled distal AP-1 cis-element of the hSERTp2. DNA-protein complexes in response to EGF treatment were competed by excess of unlabeled probe (lane 4). Excess of cold oligonucleotides representing the AP-1 consensus sequence (125-fold excess, lane 5; 150-fold excess, lane 6) competed out the increase in binding observed in EGF-treated nuclear extracts (lane 3). Addition of c-jun antibody abolished the increase in binding in response to EGF treatment (lane 7). These data indicate that c-jun binds to the potential AP-1 cis-elements of hSERTp2. A representative blot is shown.