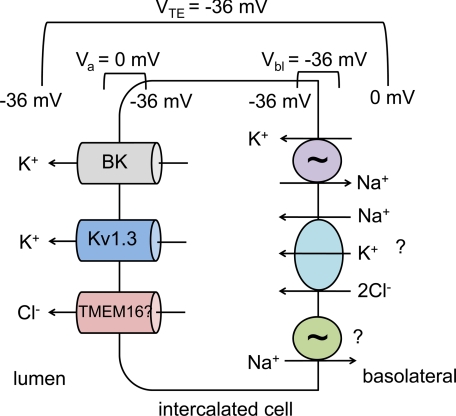
Fig. 1.
Model of intercalated cell potassium secretion. Due to a large basolateral chloride conductance (not shown), the basolateral membrane potential (Vbl) of the intercalated cell is close to the equilibrium potential for chloride, approximately −36 mV. Due to electrogenic sodium reabsorption through the epithelial sodium channel (ENaC) in neighboring principal cells (not shown), a transepithelial potential (VTE) is generated. If this is about the same as Vbl, the apical membrane potential (Va) of the intercalated cell will be ∼0 mV (see Ref. 50 for a more detailed discussion). This relatively depolarized Va results in a higher open probability for the voltage-gated potassium channels BK and Kv1.3 and provides a strong electric driving force for potassium secretion. By coupling potassium secretion to chloride secretion, a sodium-independent component of potassium secretion can be achieved. An apical chloride channel has yet to be identified, but an attractive hypothesis is that a member of the voltage- and calcium-dependent TMEM16 family could provide this function. On the basolateral side, potassium uptake may occur through the Na-K-ATPase, but because this is present at only low levels in intercalated cells, additional uptake may occur through Na+-K+-2Cl− cotransporter isoform 1 (NKCC1). If Na-K-ATPase is limiting, the cotransported sodium could be recycled either through the ouabain-insensitive Na-ATPase, or through other unidentified mechanisms, such as a Na-HCO3− cotransporter (not shown).