Abstract
The fact that certain tumors exhibit a predilection for metastasis to specific organs has been recognized for well over a century now. An extensive body of clinical data and experimental research has confirmed Stephen Paget's original “seed and soil” hypothesis that proposed the organ-preference patterns of tumor metastasis are the product of favorable interactions between metastatic tumor cells (the “seed”) and their organ microenvironment (the “soil”). Indeed, many of first-line therapeutic regimens currently in use for the treatment of human cancer are designed to target cancer cells (such as chemotherapy) and also to modulate the tumor microenvironment (such as anti-angiogenic therapy). While some types of tumors are capable of forming metastases in virtually every organ in the body, the most frequent target organs of metastasis are bone, brain, liver, and the lung. In this review, we discuss how tumor-stromal interactions influence metastasis in each of these organs.
Keywords: Metastasis, Organ Microenvironment, Astrocytes
Introduction
Current estimates regarding the global incidence of cancer predict that by the year 2020, the number of new cancer cases diagnosed each year will increase to 15 million and that the disease will be responsible for more than 12 million deaths.1 Despite recent advances in surgical techniques, radiotherapy, and the development of molecularly targeted therapies, most deaths due to cancer result from the progressive growth of metastases that are resistant to current therapies.2 Metastases originate from a selected subpopulation of cells that reside in a biologically heterogeneous primary tumor.3 Fully competent metastatic cells undergo an extremely high rate of spontaneous mutation in comparison to benign cells and hence, have a greater tendency to undergo rapid phenotypic diversification and become resistant to various therapeutic modalities.4 The development of improved therapies for metastasis is therefore one of the primary goals of cancer research.
The process of cancer metastasis consists of a series of sequential steps that begins when tumor cells detach from the extracellular matrix and invade the surrounding tissue. Localized proteolysis at the tumor cell-basement membrane interface signifies the transition from a benign carcinoma in situ to a malignant invasive tumor. Tumor cells migrate toward a vascular blood supply and penetrate thin-walled vessels in order to gain access to the systemic circulation. Tumor cells circulate as small aggregates and arrest in distal microvascular beds by passive mechanical or active mechanisms. Tumor cell extravasation may occur by multiple mechanisms. Adherent tumor cells may migrate across intercellular junctions between adjacent endothelial cells (paracellular route) or they may penetrate through the body of a single endothelial cell (transcellular route).5 Some tumor cells secrete products that stimulate endothelial cell retraction,6 whereas other tumor cells continue to divide within the vessel lumen and extravasation occurs when the vessel becomes ruptured by the expanding mass.7 Recent studies suggest that a distinct population of CD11b+ macrophages may recognize emigrating tumor cells and assist them with the invasion process.8 After gaining access to the underlying tissue parenchyma, tumor cells establish reciprocal signaling networks with stromal cells to promote their growth. To offset increasing metabolic demands associated with unrestrained cell division, tumor cells synthesize proangiogenic proteins that instruct adjacent microvascular endothelial cells to form new vascular networks (i.e., angiogenesis). Metastatic cells can repeat the entire sequence of events to produce additional metastases. Experimental evidence indicates that circulating tumor cells can also colonize their tumors of origin, in a process referred to as “tumor self-seeding”.9 The self-seeding process appears to select for cancer cell populations that are more aggressive than the majority of cells in a primary tumor and may help to explain local recurrence following tumor excision.9
Metastasis is regarded as a highly inefficient process in that less than 0.01% of circulating tumor cells eventually succeeds in forming secondary tumor growths.10 Studies examining the individual steps in the metastatic process determined that initiating cell growth in secondary organs is the most challenging step for disseminating cells.11 Some tumor cells exit the cell cycle and remain dormant in secondary organs,12 while others are incapable of triggering the angiogenic switch necessary for tumor expansion.13 The fate of the metastatic process is determined by a complex series of interactions between metastatic cells and their organ microenvironment.
Seed and Soil Hypothesis
In 1889, Stephen Paget14 examined postmortem data that had been assembled from 735 women with breast cancer and noted that the organ distribution of metastases in these patients was nonrandom. Instead, Paget suggested that metastasis is not due to chance events, but rather that some tumor cells (the “seed”) grew preferentially in the microenvironment of select organs (the “soil”) and that metastases resulted only when the appropriate seed was implanted in its suitable soil.14 Paget's assertion that the microenvironment plays a critical role in regulating the growth of metastases is supported by several experimental studies. Kinsey15 implanted small fragments of different organs in ectopic locations in syngeneic mice and demonstrated that lung-homing melanoma cells metastasized to normal lung and ectopically placed lung, but not to any other tissues. Schackert and Fidler16 extended this concept by demonstrating that some tumor cells selectively metastasize to specific regions within a given organ. Greene and Harvey17 were among the first to show that the adhesive interaction that formed between tumor cells and the luminal surface of the microvascular endothelium might be responsible for determining the localization of metastases. Auerbach and colleagues18 validated Green's proposal by demonstrating that tumor cells preferentially adhere to the microvasculature of their respective target organ.
Despite its appeal, Paget's proposal was not universally accepted and was contested by others19 who declared that the primary factor that determined the patterns of tumor metastasis was the anatomy of vascular and lymphatic drainage from the site of the primary tumor (i.e., anatomical/mechanical hypothesis). This hypothesis maintains that tumor cells follow the circulatory route draining from the primary tumor and arrest nonspecifically in the first organ encountered. Proponents of this theory argue that since the first organ encountered is the principal site of tumor cell arrest, it will possess the highest number of metastases. A body of evidence also supports the proposition that anatomical and mechanical factors are important considerations in determining the metastatic patterns of several types of tumors. For example, the liver is a common site of haematogenous metastases for tumors arising in the gastrointestinal tract due to the unique venous drainage that takes place through the portal venous system.
The prevailing consensus is one in which neither the “seed and soil” nor the anatomical-mechanical hypothesis needs to be mutually exclusive and the extent to which either mechanism (or both) is operational depends on the tumor under investigation. In the following sections, we discuss the role of tumor-stroma interactions in metastasis to different organs.
Bone metastasis
More than 350,000 individuals are reported to die from bone metastasis each year.20 Breast cancer and prostate cancer are the most common carcinomas to develop bone metastases, with an incidence of 65–75% and 68%, respectively. Carcinomas of the lung and kidney metastasize to the bone in approximately 40% of cases. Unique characteristics of the bone niche provide homing signals to these cancer cells and the biochemical (e.g., cytokines, growth factors) and physical (e.g., acidic pH, high extracellular calcium concentration) properties of the bone provide a microenvironment that is advantageous for tumor cell growth.21 The pathophysiology of bone metastasis is complex and involves many different cell populations and several regulatory proteins.
Studies suggest that bone-derived chemokines, such as osteopontin,22 osteonectin,23 and stromal-derived factor-1 (SDF-1:CXCL12),24 play a role in the trafficking of breast cancer cells and prostate cancer cells to the bone. CXCL12 is expressed by stromal cells in target organs of breast cancer metastasis (bone, brain, liver, lung, lymph node), but not in other tissues.25 CXCR4, the receptor for CXCL12, was recently identified as a critical determinant in the gene expression signature of bone-colonizing breast cancer cells.26 Activation of CXCR4 expressed on breast cancer cells stimulates a number of cellular processes involved in metastasis (e.g., pseudopodia formation, invasion, migration).27 CXCR4 also signals for integrin activation, which increases the affinity of tumor cells for microvascular endothelial cells.28
Pathologic bone remodeling is a frequent characteristic of advanced breast cancer and is the result of paracrine signaling networks between breast cancer cells and stromal cells. As many as 80% of patients with stage IV breast cancer have evidence of osteolytic bone metastasis.29 Accumulating evidence suggests that parathyroid hormone related peptide (PTHrP) plays a major role in the bone destruction observed during breast cancer metastasis. Studies show that 90% of breast cancer bone metastases express parathyroid hormone related peptide (PTHrP), whereas only 17% of metastases in non-bone organs express the protein.30 PTHrP stimulates stromal cells and osteoblasts to increase their production of receptor activator of nuclear factor κB ligand (RANKL), which then interacts with RANK expressed on osteoclast precursor cells and promotes their differentiation and activation.31 Bone resorption releases TGF-β from the bone matrix, which binds to its receptor on tumor cells and activates a positive feedback loop by signaling for increased production of PTHrP from tumor cells.32 The proangiogenic and immunosuppressive properties of TGF-β may also contribute to metastatic progression. In a preclinical model of breast cancer, neutralizing antibodies directed against PTHrP were found to abrogate osteolytic lesions.33 However, targeting of PTHrP may not be sufficient to control osteolytic bone destruction as recent studies have identified several PTHrP-independent osteolytic pathways.34
Prostate cancer spread to the bone involves both anatomical/mechanical components (i.e., Batson venous plexus) and site-specific molecular interactions. Metastatic prostate cancer cells adhere more avidly to bone marrow-derived endothelial cells in comparison to endothelial cells harvested from non-target organs.35 This binding is mediated by the tetrasaccharide sialyl LewisX (sLeX) antigen expressed on prostate cancer cells and its receptor, E-selectin, that is constitutively expressed on bone endothelial cells.36 E-selectin expression is restricted to activated endothelial cells in most vascular beds and its appearance requires cytokine simulation and protein synthesis.37 However, E-selectin is constitutively expressed on bone endothelial cells where it regulates the recirculation of hematopoietic progenitor cells to the bone.38
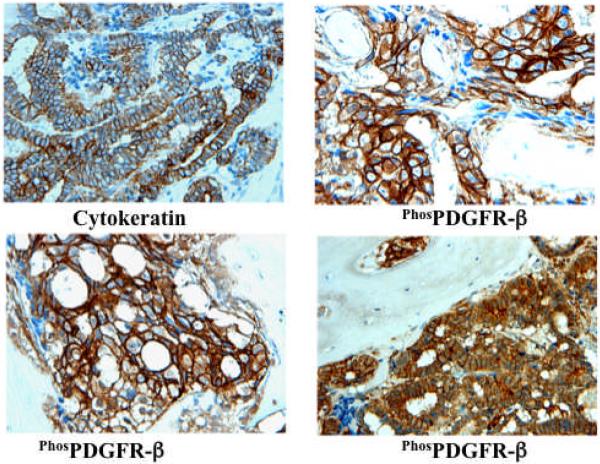
Studies examining the patterns of metastasis in advanced prostate cancer indicate that metastasis to the bone and lymph nodes occur in over 80% of cases. To study tumor-stromal interactions in prostate cancer metastasis, we developed an orthotopic model of hormone-refractory prostate cancer metastasis by implanting androgen-independent PC3-MM2 cancer cells into the bone cortex of nude mice. An examination of these tumors suggested that the platelet-derived growth factor receptor-β (PDGFR-β) signaling pathway may be particularly important for metastatic progression given that PDGFR-β was expressed in its activated form on both tumor and stroma (tumor-associated endothelial cells).39 Moreover, we found an identical pattern of phosphorylated PDGFR-β expression in bone biopsy specimens from prostate cancer patients (Figure 1). Treatment of mice with the small molecule tyrosine kinase inhibitor imatinib plus taxol prevented the phosphorylation of PDGFR-β on tumor cells and tumor-associated endothelial cells and led to significant apoptosis of both of these populations of cells. The combination therapy resulted in smaller tumors, fewer lymphatic metastases, and the preservation of bone structure. Additional studies performed on monolayers of bone microvascular endothelial cells indicated that these cells expressed significant levels of PDGFR-β, and that they responded to stimulation with PDGF ligand by increasing their cell division, activating Akt and ERK1/2 signaling, and upregulating Bcl-2.40 Blockade of PDGFR-β signaling on the bone microvascular endothelial cells with imatinib inhibited activation of the downstream targets, while the coadministration of imatinib and taxol resulted in induction of procaspase-3 and significant apoptosis.40
Figure 1.
Expression of phosphorylated PDGFR-β in clinical bone marrow samples that were collected from patients with prostate cancer. Tumor foci were identified by positive staining for cytokeratin 18 (upper left panel). Additional specimens were labeled with an antibody specific for the phosphorylated form of the PDGFR-β (remaining panels).
The abovementioned studies suggested that tumor-associated endothelial cells in the bone might be the primary target for the combination therapy of imatinib and taxol. To test this hypothesis, we selected multidrug-resistant PC3-MM2 prostate cancer cells (PC3-MM2-MDR cells) that were 70 times more resistant to taxol than parental cells and were also refractory to the combination of taxol and imatinib in vitro.41 Phosphorylated PDGFR-β was expressed by tumor cells and tumor-associated endothelial cells in PC3-MM2-MDR tumors growing in the bones of nude mice. We also found that the PC3-MM2-MDR tumors were sensitive to the systemic administration of taxol and imatinib (but not to taxol alone) and that the combination therapy significantly reduced the tumor size and incidence of lymphatic metastasis.41 A time course study showed that the first wave of apoptosis occurs strictly on tumor-associated endothelial cells, which allowed us to conclude that the ensuing tumor cell death was most likely due to an insufficient vascular supply.
Investigations conducted by other laboratories also implicated the PDGFR-β signaling pathway in prostate tumor progression. Indeed, PDGFR-β was identified as one of five genes that predicted recurrence following prostatectomy.42 Therefore, a neoadjuvant study was conducted to evaluate hormone ablation, docetaxel and imatinib in men with high-risk localized prostate cancer.43 Unfortunately, the results of that study revealed a high frequency of preoperative non-hematological toxicity, no defined pathological complete responses and a 3-year progression-free survival that was similar to historical controls.43 The disparity between the preclinical findings and the clinical results emphasize some of the challenges that investigators face when attempting to model the clinical setting. One of the more important limitations of experimental tumor models is that they simply cannot accurately model the chronic evolution of the human disease.44 For example, mathematical modeling of the genetic evolution of human pancreatic cancer revealed that at least a decade passes between the occurrence of the initiating mutation and the birth of the parental, non-metastatic founder cell and that at least five more years are required for the acquisition of metastatic ability.45 Continuing advances in the generation of genetically engineered mice, which permit tumors to develop de novo and progress within the natural microenvironment, may lead to improvements in predicting clinical efficacy of cancer therapies.46
Lung metastasis
The lung is the second most common site for the occurrence of metastasis. Tumors that originate from the breast, bladder, colon, kidney, head and neck, and skin (melanoma) all have a tendency to metastasize to the lung. The dense vascular surface area of the lung makes it a particularly attractive microenvironment for supporting the outgrowth of metastases. Measurements of the adult pulmonary vascular surface area indicate that it occupies as much as 100 square meters,47 which is significantly higher than that of any other organ.
Experimental models of melanoma metastasis in which tumor cells are inoculated directly into the venous circulation showed that the frequency of pulmonary metastasis is significantly enhanced when animals are treated with proinflammatory cytokines prior to injection of tumor cells.48, 49 The enhanced metastatic burden observed in these models was due to a direct increase in expression of the inducible endothelial cell adhesion molecule, vascular cell adhesion molecule-1 (VCAM-1). However, data derived from spontaneous tumor models of melanoma metastasis that require successful completion of each step of the metastatic process showed that primary melanoma tumors do no stimulate enhanced levels of VCAM-1 on the lung microvasculature.50 Given the extremely small diameter of lung capillaries (4 μm) and relatively large diameter of tumor cells (20 μm), it is conceivable that the upregulation of adhesion molecules may not be a prerequisite for tumor cell arrest in this organ.
However, there is evidence that suggests some tumors transmit prometastatic signals to the lung that prime the metastatic soil for the influx of tumor cells. Hiratsuka et al.,51 reported that experimental tumors and some human tumors activate vascular endothelial growth factor-1 (VEGFR-1) on distal lung endothelial cells in order to increase their expression of the matrix metalloproteinase MMP-9 before tumor cell spread. MMP-9 produced in lung during the premetastatic phase was shown to make the tissue much more receptive to tumor cell invasion. Similarly, Kaplan and coworkers52 reported that primary tumors produce factors that stimulate lung fibroblasts to increase their expression of fibronectin. The elevated level of fibronectin provides a chemotactic gradient for VEGFR-1 positive hematopoietic progenitor cells that migrate to the lung and form a premetastatic niche. The clustered bone marrow hematopoietic cells then release chemotactic cytokines that provide directional information for metastasizing tumor cells. It remains unclear whether tumor cells rely on premetastatic signaling to modify the microenvironment of other target organs of metastasis.
Stromal products produced in lung environment can significantly enhance the resistance of pulmonary metastases to chemotherapeutic agents. Willmanns et al.,53 found that CT-26 colon cancer cells residing in the lungs of syngeneic mice were refractory to the effects of doxorubicin treatment, whereas the same cells implanted in the skin of mice were highly sensitive to the drug. The enhanced resistance to drug in the lung microenvironment was due to an upregulation of P-glycoprotein in the cells. P-glycoprotein is a member of the superfamily of ATP-binding cassette transporters and functions as an energy-dependent efflux protein to expel a variety of toxic compounds from cells, including many chemotherapeutic drugs.54
Recent studies have shown that chronic stress may have a profound impact on the metastatic process.55 In a mouse model of ovarian cancer, the increased levels of catecholamines resulting from chronic behavioral stress were found to signal for an increase in the tumor vascular surface area, which led to a significant increase in peritoneal metastases.56 Similarly, in a breast cancer model, stress-induced neuroendocrine activation resulted in a 37-fold increase in the formation of lung metastases.57 In that report, macrophages were identified as the intratumoral target of sympathetic nervous system signaling and these cells modified the primary tumor microenvironment by enhancing their expression of immunosuppressive and prometastic molecules.57 Additional research is needed to determine whether stress modifies target organs of metastasis and renders these tissues more susceptible to colonization.
Liver metastasis
The liver is a frequent site of metastasis for several tumors including, cutaneous melanoma, lung, colorectal, breast, and neuroendocrine tumors. Choroidal melanoma frequently metastasizes to the liver and this relationship cannot be satisfactorily explained by anatomical-mechanical principles. About 40–50% of women with metastatic breast cancer have metastasis to the liver during the course of the disease.58 In general, the prognosis for patients with liver metastases is poor. Studies have shown that expression levels of sLex and sLea selectin ligands on colorectal cancer cells correlates with their metastatic potential.59 Colorectal cancer cells were found to use sLex and sLea to support their adhesion to cytokine-induced E-selectin expressed on vascular endothelial cells.60 Brodt and coworkers61 noted that mice treated with anti-E-selectin monoclonal antibodies formed significantly fewer experimental liver metastases following intrasplenic injection of Lewis lung carcinoma variants. Khatib et al.,62 omitted exogenous cytokine administration from their study and demonstrated that the entry of HT-59 carcinoma cells into the liver elicited endogenous IL-1 and TNF-α production, the kinetics of which correlated with the induction of E-selectin on hepatic sinusoidal endothelial cells.
An expanding body of evidence suggests that the transforming growth α (TGFα)/epidermal growth factor receptor (EGFR) signaling pathway plays a critical role in colon cancer metastasis. Metastatic human colon cancer cells express five times more EGFR when compared to non-metastatic cells.63 In a preclinical model of colorectal cancer, we64 found that the inclusion of an EGFR inhibitor to therapy significantly reduced the mass of the primary tumor and decreased the frequency of lymphatic metastasis. Cetuximab, a chimeric monoclonal antibody that blocks ligand binding to EGFR demonstrated modest activity as a single agent in metastatic colorectal cancer patients.65 In patients with irinotecan-refractory colorectal cancer, the combination of cetuximab and irinotecan resulted in a 22% response rate.66
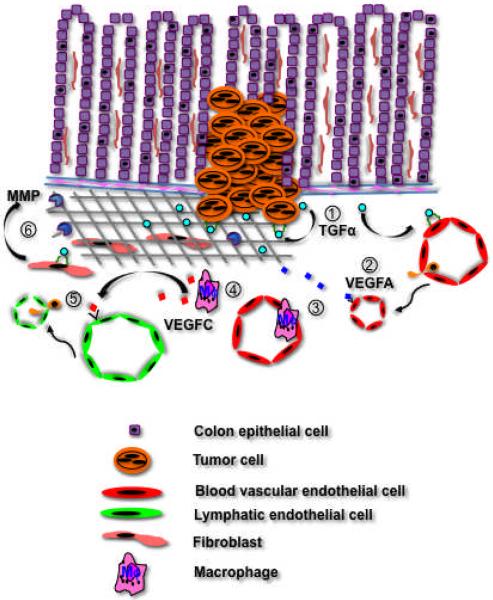
To determine how the TGFα/EGFR signaling pathway contributes to colon cancer metastasis, we implanted KM12C colon cancer cell clones expressing high (C9) or negligible (C10) levels of TGFα into the cecal walls of nude mice.67 Only C9 tumors formed autocrine and paracrine EGFR networks and had a microenvironment that was enriched in VEGF, IL-8, MMP-2 and -9. C9 tumors also recruited a macrophage population that co-expressed F4/80 and LYVE-1 and produced VEGFC. The lymphatic vascular density of C9 tumors was 3-fold higher than that of C10 tumors. Moreover, C9 tumor cells metastasized to regional lymph nodes in 100% of mice and to the liver in 50% of mice, whereas C10 tumors cells spread to lymph nodes in 10% of mice and were incapable of generating liver metastases. These data suggest that activation of TGFα/EGFR paracrine signaling networks in colon tumors creates a microenvironment that is conducive for metastasis (Figure 2).
Figure 2.
Colon cancer cells produce TGFα that acts in an autocrine manner to promote tumor growth and in a paracrine fashion to activate angiogenic programs in resident endothelial cells (1). This signaling is mediated by the EGFR that is expressed on both cell populations. Tumor cells express VEGFA that reinforces the angiogenic response (2), and the expansion in the tumor vascular surface area increases the likelihood of haematogenous metastasis. VEGFA is also a potent chemoattractant for macrophages (3), which secrete VEGFC in the tumor microenvironment (4). VEGFC binds to VEGFR-3 expressed on lymphatic endothelial cells and stimulates lymphangiogenesis (5), which facilitates the spread of tumor cells to regional lymph nodes. TGFα produced by colon tumor cells binds to the myofibroblast EGFR, which respond by entering the cell cycle and increasing their production of MMP (6). MMP degradation of the extracellular matrix enhances tumor cell invasion.
Brain metastasis
In the USA, as many as 170,000 new cases of brain metastases occur each year, which is ten times the number of patients diagnosed with malignant primary brain tumors.68 In fact, more than 40% of cancer patients develop brain metastasis; specifically, nearly 50% of patients with lung cancer, >25% of patients with breast cancer, and 20% of patients with melanoma.69–71 The incidence of brain metastasis may be increasing as cancer patients are living longer as a result of improved treatment and also because the incidence of lung cancer and melanoma continue to rise.72 The progressive growth of metastasis in the brain is frequently associated with the terminal stage of the disease. The therapeutic approach for patients with brain metastases is dependent on the number and location of metastases, on the biology of the primary tumor, and on the extent of systemic disease.73 The median survival for untreated patients is 1–2 months, which may be extended to 6 months with conventional radiotherapy and chemotherapy.74
Recent investigations are beginning to provide insight into the cellular and molecular mechanisms that facilitate the recruitment and retention of tumor cells to the CNS. Bos et al.,75 performed comparative genome-wide expression analysis on breast cancer cell brain metastatic variants and identified the cyclooxygenase COX2, the EGFR ligand HB-EGF, and the α2,6-sialyltranferase ST6GALNAC5, as critical mediators of extravasation through the blood-brain barrier. Lee et al.,76 examined breast cancer cell migration through a human blood-brain barrier model and determined that CCL12 increased the permeability brain endothelial cell monolayers and promoted MDA-MB-231 transendothelial cell migration. We found that blockade of VCAM-1 expressed on lymphatic endothelial cells significantly reduced the ability of B16-F1 melanoma cells to adhere to lymphatic endothelial cells, whereas the same treatment had no effect on tumor cell adhesion to brain endothelial cells.77 This latter study suggests that melanoma cells may rely on different receptor-ligand pairs to facilitate their adhesion to vascular endothelium of different tissues.
Once tumor cells leave the systemic circulation, the primary determinant that governs their survival is their proximity to a vascular blood supply. In autochthonous human lung cancer brain metastases, dividing tumor cells are usually located within 75 μm of the nearest blood vessel, whereas apoptotic tumor cells are located 160–170 μm from the nearest blood vessel.78 These measurements correlate with the diffusion coefficient of oxygen in tumor tissue, which is approximately 120 μm.79 Fidler et al.,78 studied the vascular patterns of several different types of experimental brain metastases and noted that the mean vessel density (MVD) of the tumors was 15–20 times less than the MVD of the normal brain parenchyma. While others have reported significant reductions in the MVD of intracranial tumors in comparison to corresponding normal tissue,80 it should be noted that considerable heterogeneity exists in the angiogenic response of different tumors.81 Pioneer studies evaluating the tumor vascular bed demonstrated that the vascular space becomes progressively smaller as the tumor increases in mass.82 Tannock has attributed the reduction of tumor vascular surface in growing tumors to a difference in the turnover time between endothelial cells (50 to 60 hours) and tumor cells (22 hours).83
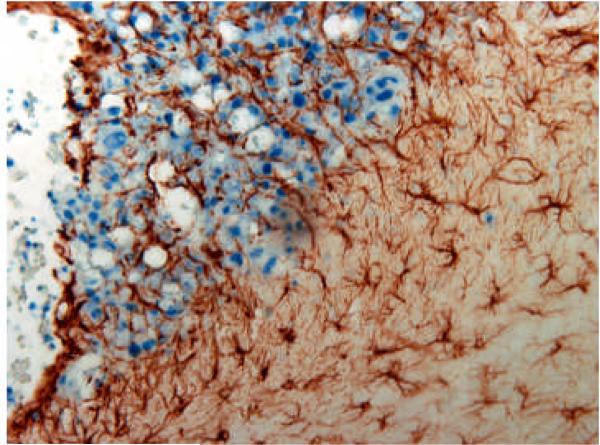
Over recent years, there has been a growing appreciation that stromal cells not only contribute to malignant progression, but that they also play a key role in mediating acquired resistance to therapy. Aggressive management of brain metastases usually extends survival to only 4 to 6 months.84 The limited efficacy of current treatment of brain metastasis is primarily due to chemoresistance.85 The blood-brain barrier is breached in metastases as evidenced by magnetic resonance image, which show that more than 70% of brain metastases have leakage of contrast agent from blood vessels86 and hence, brain metastases are exposed to therapeutic agents. Recently, Lin et al.,87 demonstrated that astrocytes protect tumor cells from cytotoxicity induced by chemotherapeutic drugs. The investigators determined that the chemotherapeutic effect was dependent on physical contact and gap junction communication between astrocytes and tumor cells. Figure 3 shows the reactive astrogliosis that is characteristic of brain metastasis.
Figure 3.
Tumor cell-astrocyte interaction in experimental 3LL brain metastases. Brain tissues were sectioned and incubated with an antibody directed against GFAP, which labels reactive astrocytes. Reactive astrocytes (brown) encircle and infiltrate the tumor mass (blue). No GFAP-positive astrocytes were detected in the same location of the brain in non-tumor-bearing mice (data not shown).
Therapeutic targeting of advanced/metastatic tumors and the organ microenvironment
Efforts directed toward targeting the tumor microenvironment for therapy of advanced cancers have largely focused on the angiogenic blood vessels that support tumor growth. The proangiogenic cytokine VEGF is overexpressed in the majority of human tumors and is a central regulator of pathologic angiogenesis.88 Several therapeutic agents designed to inhibit VEGF-induced angiogenesis are now in clinical use including, monoclonal antibodies that block VEGF (i.e., bevacizumab), and small molecule inhibitors of the VEGFR-2 tyrosine kinase (e.g., sorafinib, sunitinib). The addition of bevacizumab to standard chemotherapy was shown to prolong progression-free survival and overall survival in patients with advanced non-small cell lung cancer (NSCLC) and colon cancer.89, 90 However, to date, the dramatic results reported in preclinical studies have not been reproduced in the clinical setting. For example, the current first-line regimen for advanced/metastatic non-small cell lung cancer (bevacizumab plus carboplatin and paclitaxel) increases overall survival by only two months when compared to chemotherapy alone (12.3 months vs 10.3 months).89 In colon cancer, the addition of bevacizumab to standard therapy increased the median duration of survival by 4.7 months.90 A rapidly expanding body of evidence indicates that both tumor cells and host cells contribute to the resistance of therapies that are designed to block the VEGF signaling pathway.91–93 Identifying and overcoming the mechanisms that mediate therapeutic resistance will likely yield improved clinical outcomes.
Conclusions
The outcome of metastasis is determined by a number of complex interactions between malignant cells (the “seed”) and their organ microenvironment (the soil”). Over recent years, there has been an appreciable increase in our understanding of the crosstalk that occurs between these two compartments on the systemic, cellular, and molecular bases. Continued investigations of the mechanisms that mediate site-specific metastasis will likely lead to the identification of new targets for therapy.
Acknowledgments
We thank Arminda Martinez for expert assistance in the preparation of this manuscript.
Grant Sponsors: Cancer Center Support Core Grant CA16672 and Grant 1U54CA143837-01 from the National Cancer Institute, National Institutes of Health.
Abbreviations
- VEGF
Vascular Endothelial Cell Growth Factor
- MMP
Matrix Metalloproteinase
References
- 1.World Health Orgaization Press Release WHO/27/IARC/145. World Health Organization; Geneva: 2003. The World Cancer Report - the major findings. [Google Scholar]
- 2.Langley RR, Fidler IJ. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr Rev. 2007;28:297–321. doi: 10.1210/er.2006-0027. [DOI] [PubMed] [Google Scholar]
- 3.Fidler IJ. The pathogenesis of cancer metastasis: the `seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 4.Talmadge JE, Benedict K, Madsen J, Fidler IJ. Development of biological diversity and susceptibility to chemotherapy in murine cancer metastases. Cancer Res. 1984;44:3801–5. [PubMed] [Google Scholar]
- 5.Kawaguchi T, Nakamura K. Analysis of the lodgement and extravasation of tumor cells in experimental models of hematogenous metastasis. Cancer Metastasis Rev. 1986;5:77–94. doi: 10.1007/BF00046424. [DOI] [PubMed] [Google Scholar]
- 6.Honn KV, Tang DG, Grossi I, Duniec ZM, Timar J, Renaud C, Leithauser M, Blair I, Johnson CR, Diglio CA, et al. Tumor cell-derived 12(S)-hydroxyeicosatetraenoic acid induces microvascular endothelial cell retraction. Cancer Res. 1994;54:565–74. [PubMed] [Google Scholar]
- 7.Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med. 2000;6:100–2. doi: 10.1038/71429. [DOI] [PubMed] [Google Scholar]
- 8.Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, Lang RA, Pollard JW. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4:e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, Massague J. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–26. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fidler IJ. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2'-deoxyuridine. J Natl Cancer Inst. 1970;45:773–82. [PubMed] [Google Scholar]
- 11.Chambers AF, MacDonald IC, Schmidt EE, Koop S, Morris VL, Khokha R, Groom AC. Steps in tumor metastasis: new concepts from intravital videomicroscopy. Cancer Metastasis Rev. 1995;14:279–301. doi: 10.1007/BF00690599. [DOI] [PubMed] [Google Scholar]
- 12.Barkan D, Green JE, Chambers AF. Extracellular matrix: a gatekeeper in the transition from dormancy to metastatic growth. Eur J Cancer. 2010;46:1181–8. doi: 10.1016/j.ejca.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hedley BD, Chambers AF. Tumor dormancy and metastasis. Adv Cancer Res. 2009;102:67–101. doi: 10.1016/S0065-230X(09)02003-X. [DOI] [PubMed] [Google Scholar]
- 14.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 15.Kinsey DL. An experimental study of preferential metastasis. Cancer. 1960;13:674–6. doi: 10.1002/1097-0142(196007/08)13:4<674::aid-cncr2820130405>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Schackert G, Fidler IJ. Site-specific metastasis of mouse melanomas and a fibrosarcoma in the brain or meninges of syngeneic animals. Cancer Res. 1988;48:3478–84. [PubMed] [Google Scholar]
- 17.Greene HS, Harvey EK. The relationship between the dissemination of tumor cells and the distribution of metastases. Cancer Res. 1964;24:799–811. [PubMed] [Google Scholar]
- 18.Auerbach R, Lu WC, Pardon E, Gumkowski F, Kaminska G, Kaminski M. Specificity of adhesion between murine tumor cells and capillary endothelium: an in vitro correlate of preferential metastasis in vivo. Cancer Res. 1987;47:1492–6. [PubMed] [Google Scholar]
- 19.Ewing J. Neoplastic diseases. 3rd ed. WB Saunders; Philadelphia: 1928. [Google Scholar]
- 20.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 21.Guise T. Examining the metastatic niche: targeting the microenvironment. Semin Oncol. 2010;37(Suppl 2):S2–14. doi: 10.1053/j.seminoncol.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim T, Leong I, Sanchez-Sweatman O, Khokha R, Sodek J, Tenenbaum HC, Ganss B, Cheifetz S. Expression of bone sialoprotein and osteopontin in breast cancer bone metastases. Clin Exp Metastasis. 2000;18:253–60. doi: 10.1023/a:1006754605901. [DOI] [PubMed] [Google Scholar]
- 23.Jacob K, Webber M, Benayahu D, Kleinman HK. Osteonectin promotes prostate cancer cell migration and invasion: a possible mechanism for metastasis to bone. Cancer Res. 1999;59:4453–7. [PubMed] [Google Scholar]
- 24.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–7. [PubMed] [Google Scholar]
- 25.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 26.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–49. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 27.Luker KE, Luker GD. Functions of CXCL12 and CXCR4 in breast cancer. Cancer Lett. 2006;238:30–41. doi: 10.1016/j.canlet.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 28.Cardones AR, Murakami T, Hwang ST. CXCR4 enhances adhesion of B16 tumor cells to endothelial cells in vitro and in vivo via beta(1) integrin. Cancer Res. 2003;63:6751–7. [PubMed] [Google Scholar]
- 29.Kozlow W, Guise TA. Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia. 2005;10:169–80. doi: 10.1007/s10911-005-5399-8. [DOI] [PubMed] [Google Scholar]
- 30.Powell GJ, Southby J, Danks JA, Stillwell RG, Hayman JA, Henderson MA, Bennett RC, Martin TJ. Localization of parathyroid hormone-related protein in breast cancer metastases: increased incidence in bone compared with other sites. Cancer Res. 1991;51:3059–61. [PubMed] [Google Scholar]
- 31.Yoneda T, Hiraga T. Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochem Biophys Res Commun. 2005;328:679–87. doi: 10.1016/j.bbrc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 32.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–64. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 33.Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF, Yoneda T, Mundy GR. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest. 1996;98:1544–9. doi: 10.1172/JCI118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen CR, Manova-Todorova K, Blasberg R, Gerald WL, Massague J. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci U S A. 2005;102:13909–14. doi: 10.1073/pnas.0506517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lehr JE, Pienta KJ. Preferential adhesion of prostate cancer cells to a human bone marrow endothelial cell line. J Natl Cancer Inst. 1998;90:118–23. doi: 10.1093/jnci/90.2.118. [DOI] [PubMed] [Google Scholar]
- 36.Barthel SR, Wiese GK, Cho J, Opperman MJ, Hays DL, Siddiqui J, Pienta KJ, Furie B, Dimitroff CJ. Alpha 1,3 fucosyltransferases are master regulators of prostate cancer cell trafficking. Proc Natl Acad Sci U S A. 2009;106:19491–6. doi: 10.1073/pnas.0906074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eppihimer MJ, Wolitzky B, Anderson DC, Labow MA, Granger DN. Heterogeneity of expression of E- and P-selectins in vivo. Circ Res. 1996;79:560–9. doi: 10.1161/01.res.79.3.560. [DOI] [PubMed] [Google Scholar]
- 38.Katayama Y, Hidalgo A, Furie BC, Vestweber D, Furie B, Frenette PS. PSGL-1 participates in E-selectin-mediated progenitor homing to bone marrow: evidence for cooperation between E-selectin ligands and alpha4 integrin. Blood. 2003;102:2060–7. doi: 10.1182/blood-2003-04-1212. [DOI] [PubMed] [Google Scholar]
- 39.Uehara H, Kim SJ, Karashima T, Shepherd DL, Fan D, Tsan R, Killion JJ, Logothetis C, Mathew P, Fidler IJ. Effects of blocking platelet-derived growth factor-receptor signaling in a mouse model of experimental prostate cancer bone metastases. J Natl Cancer Inst. 2003;95:458–70. doi: 10.1093/jnci/95.6.458. [DOI] [PubMed] [Google Scholar]
- 40.Langley RR, Fan D, Tsan RZ, Rebhun R, He J, Kim SJ, Fidler IJ. Activation of the platelet-derived growth factor-receptor enhances survival of murine bone endothelial cells. Cancer Res. 2004;64:3727–30. doi: 10.1158/0008-5472.CAN-03-3863. [DOI] [PubMed] [Google Scholar]
- 41.Kim SJ, Uehara H, Yazici S, Busby JE, Nakamura T, He J, Maya M, Logothetis C, Mathew P, Wang X, Do KA, Fan D, et al. Targeting platelet-derived growth factor receptor on endothelial cells of multidrug-resistant prostate cancer. J Natl Cancer Inst. 2006;98:783–93. doi: 10.1093/jnci/djj211. [DOI] [PubMed] [Google Scholar]
- 42.Singh D, Febbo PG, Ross K, Jackson DG, Manola J, Ladd C, Tamayo P, Renshaw AA, D'Amico AV, Richie JP, Lander ES, Loda M, et al. Gene expression correlates of clinical prostate cancer behavior. Cancer Cell. 2002;1:203–9. doi: 10.1016/s1535-6108(02)00030-2. [DOI] [PubMed] [Google Scholar]
- 43.Mathew P, Pisters LL, Wood CG, Papadopoulos JN, Williams DL, Thall PF, Wen S, Horne E, Oborn CJ, Langley R, Fidler IJ, Pettaway CA. Neoadjuvant platelet derived growth factor receptor inhibitor therapy combined with docetaxel and androgen ablation for high risk localized prostate cancer. J Urol. 2009;181:81–7. doi: 10.1016/j.juro.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Ellis LM, Fidler IJ. Finding the tumor copycat. Therapy fails, patients don't. Nat Med. 2010;16:974–5. doi: 10.1038/nm0910-974. [DOI] [PubMed] [Google Scholar]
- 45.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, Velculescu VE, Kinzler KW, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–7. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Dyke T. Finding the tumor copycat: approximating a human cancer. Nat Med. 2010;16:976–7. doi: 10.1038/nm0910-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levitzky MG. Pulmonary Physiology. 2nd ed. McGraw-Hill; New York: 1986. p. 83. [Google Scholar]
- 48.Okahara H, Yagita H, Miyake K, Okumura K. Involvement of very late activation antigen 4 (VLA-4) and vascular cell adhesion molecule 1 (VCAM-1) in tumor necrosis factor alpha enhancement of experimental metastasis. Cancer Res. 1994;54:3233–6. [PubMed] [Google Scholar]
- 49.Garofalo A, Chirivi RG, Foglieni C, Pigott R, Mortarini R, Martin-Padura I, Anichini A, Gearing AJ, Sanchez-Madrid F, Dejana E, et al. Involvement of the very late antigen 4 integrin on melanoma in interleukin 1-augmented experimental metastases. Cancer Res. 1995;55:414–9. [PubMed] [Google Scholar]
- 50.Langley RR, Carlisle R, Ma L, Specian RD, Gerritsen ME, Granger DN. Endothelial expression of vascular cell adhesion molecule-1 correlates with metastatic pattern in spontaneous melanoma. Microcirculation. 2001;8:335–45. doi: 10.1038/sj/mn/7800098. [DOI] [PubMed] [Google Scholar]
- 51.Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, Shipley JM, Senior RM, Shibuya M. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 52.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilmanns C, Fan D, O'Brian CA, Bucana CD, Fidler IJ. Orthotopic and ectopic organ environments differentially influence the sensitivity of murine colon carcinoma cells to doxorubicin and 5-fluorouracil. Int J Cancer. 1992;52:98–104. doi: 10.1002/ijc.2910520118. [DOI] [PubMed] [Google Scholar]
- 54.Borst P, Schinkel AH. Genetic dissection of the function of mammalian P-glycoproteins. Trends Genet. 1997;13:217–22. doi: 10.1016/S0168-9525(97)01112-8. [DOI] [PubMed] [Google Scholar]
- 55.Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol. 2010;6:1863–81. doi: 10.2217/fon.10.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, Merritt WM, Lin YG, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–44. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 57.Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L, Sood AK, Cole SW. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–52. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diamond JR, Finlayson CA, Borges VF. Hepatic complications of breast cancer. Lancet Oncol. 2009;10:615–21. doi: 10.1016/S1470-2045(09)70029-4. [DOI] [PubMed] [Google Scholar]
- 59.Sato M, Narita T, Kimura N, Zenita K, Hashimoto T, Manabe T, Kannagi R. The association of sialyl Lewis(a) antigen with the metastatic potential of human colon cancer cells. Anticancer Res. 1997;17:3505–11. [PubMed] [Google Scholar]
- 60.Giavazzi R, Foppolo M, Dossi R, Remuzzi A. Rolling and adhesion of human tumor cells on vascular endothelium under physiological flow conditions. J Clin Invest. 1993;92:3038–44. doi: 10.1172/JCI116928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brodt P, Fallavollita L, Bresalier RS, Meterissian S, Norton CR, Wolitzky BA. Liver endothelial E-selectin mediates carcinoma cell adhesion and promotes liver metastasis. Int J Cancer. 1997;71:612–9. doi: 10.1002/(sici)1097-0215(19970516)71:4<612::aid-ijc17>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 62.Khatib AM, Kontogiannea M, Fallavollita L, Jamison B, Meterissian S, Brodt P. Rapid induction of cytokine and E-selectin expression in the liver in response to metastatic tumor cells. Cancer Res. 1999;59:1356–61. [PubMed] [Google Scholar]
- 63.Radinsky R, Risin S, Fan D, Dong Z, Bielenberg D, Bucana CD, Fidler IJ. Level and function of epidermal growth factor receptor predict the metastatic potential of human colon carcinoma cells. Clin Cancer Res. 1995;1:19–31. [PubMed] [Google Scholar]
- 64.Rebhun RB, Langley RR, Yokoi K, Fan D, Gershenwald JE, Fidler IJ. Targeting receptor tyrosine kinase on lymphatic endothelial cells for the therapy of colon cancer lymph node metastasis. Neoplasia. 2006;8:747–57. doi: 10.1593/neo.06322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saltz LB, Meropol NJ, Loehrer PJ, Sr., Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–8. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 66.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 67.Sasaki T, Nakamura T, Rebhun RB, Cheng H, Hale KS, Tsan RZ, Fidler IJ, Langley RR. Modification of the primary tumor microenvironment by transforming growth factor alpha-epidermal growth factor receptor signaling promotes metastasis in an orthotopic colon cancer model. Am J Pathol. 2008;173:205–16. doi: 10.2353/ajpath.2008.071147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nathoo N, Chahlavi A, Barnett GH, Toms SA. Pathobiology of brain metastases. J Clin Pathol. 2005;58:237–42. doi: 10.1136/jcp.2003.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1998. CA Cancer J Clin. 1998;48:6–29. doi: 10.3322/canjclin.48.1.6. [DOI] [PubMed] [Google Scholar]
- 70.Sawaya RBR, Lang FF. Metastatic Brain Tumors. Churchill-Livingstone; New York: 2001. pp. 999–1026. [Google Scholar]
- 71.Norden AD, Wen PY, Kesari S. Brain metastases. Curr Opin Neurol. 2005;18:654–61. doi: 10.1097/01.wco.0000191514.37498.2b. [DOI] [PubMed] [Google Scholar]
- 72.Conrad CA. Chemotherapy for metastatic tumors to the central nervous system. Curr Oncol Rep. 2001;3:490–4. doi: 10.1007/s11912-001-0070-z. [DOI] [PubMed] [Google Scholar]
- 73.DeAngelis LM, Posner JB. Brain Metastasis. In: Kuffe DW, Pollock RE, Weichselbaum RR, Bast RC, Gansler TS, Holland JF, Frei E, editors. Cancer Medicine. 6th ed. BC Decker Inc; Hamilton, Ontario: 2003. pp. 1227–31. [Google Scholar]
- 74.Freilich RJ, Seidman AD, DeAngelis LM. Central nervous system progression of metastatic breast cancer in patients treated with paclitaxel. Cancer. 1995;76:232–6. doi: 10.1002/1097-0142(19950715)76:2<232::aid-cncr2820760212>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 75.Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA, Massague J. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–9. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee BC, Lee TH, Avraham S, Avraham HK. Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1alpha in breast cancer cell migration through human brain microvascular endothelial cells. Mol Cancer Res. 2004;2:327–38. [PubMed] [Google Scholar]
- 77.Rebhun RB, Cheng H, Gershenwald JE, Fan D, Fidler IJ, Langley RR. Constitutive expression of the alpha4 integrin correlates with tumorigenicity and lymph node metastasis of the B16 murine melanoma. Neoplasia. 2010;12:173–82. doi: 10.1593/neo.91604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fidler IJ, Yano S, Zhang RD, Fujimaki T, Bucana CD. The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol. 2002;3:53–7. doi: 10.1016/s1470-2045(01)00622-2. [DOI] [PubMed] [Google Scholar]
- 79.Grote J, Susskind R, Vaupel P. Oxygen diffusivity in tumor tissue (DS-carcinosarcoma) under temperature conditions within the range of 20--40 degrees C. Pflugers Arch. 1977;372:37–42. doi: 10.1007/BF00582204. [DOI] [PubMed] [Google Scholar]
- 80.Viacava P, Gasperi M, Acerbi G, Manetti L, Cecconi E, Bonadio AG, Naccarato AG, Acerbi F, Parenti G, Lupi I, Genovesi M, Martino E. Microvascular density and vascular endothelial growth factor expression in normal pituitary tissue and pituitary adenomas. J Endocrinol Invest. 2003;26:23–8. doi: 10.1007/BF03345118. [DOI] [PubMed] [Google Scholar]
- 81.Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res. 2000;60:1388–93. [PubMed] [Google Scholar]
- 82.Vogel AW. Intratumoral vascular changes with increased size of a mammary adenocarcinoma: new method and results. J Natl Cancer Inst. 1965;34:571–8. [PubMed] [Google Scholar]
- 83.Tannock IF. Population kinetics of carcinoma cells, capillary endothelial cells, and fibroblasts in a transplanted mouse mammary tumor. Cancer Res. 1970;30:2470–6. [PubMed] [Google Scholar]
- 84.Soffietti R, Ruda R, Mutani R. Management of brain metastases. J Neurol. 2002;249:1357–69. doi: 10.1007/s00415-002-0870-6. [DOI] [PubMed] [Google Scholar]
- 85.McWilliams RR, Rao RD, Brown PD, Link MJ, Buckner JC. Treatment options for brain metastases from melanoma. Expert Rev Anticancer Ther. 2005;5:809–20. doi: 10.1586/14737140.5.5.809. [DOI] [PubMed] [Google Scholar]
- 86.Rowley HA, Scialfa G, Gao PY, Maldjian JA, Hassell D, Kuhn MJ, Wippold FJ, 2nd, Gallucci M, Bowen BC, Schmalfuss IM, Ruscalleda J, Bastianello S, et al. Contrast-enhanced MR imaging of brain lesions: a large-scale intraindividual crossover comparison of gadobenate dimeglumine versus gadodiamide. AJNR Am J Neuroradiol. 2008;29:1684–91. doi: 10.3174/ajnr.A1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin Q, Balasubramanian K, Fan D, Kim SJ, Guo L, Wang H, Bar-Eli M, Aldape KD, Fidler IJ. Reactive astrocytes protect melanoma cells from chemotherapy by sequestering intracellular calcium through gap junction communication channels. Neoplasia. 2010;12:748–54. doi: 10.1593/neo.10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 89.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 90.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 91.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–20. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 93.Crawford Y, Kasman I, Yu L, Zhong C, Wu X, Modrusan Z, Kaminker J, Ferrara N. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15:21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]