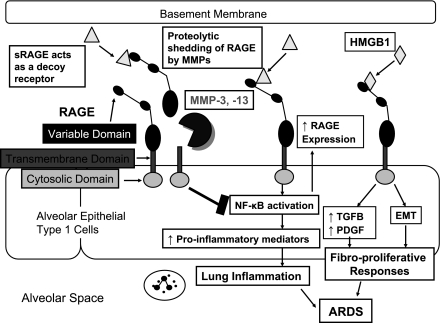
Fig 1.
Potential impact of matrix metalloproteinase (MMP)-mediated proteolytic shedding of receptor for advanced glycation end products (RAGE) on the pathogenesis of acute respiratory distress syndrome (ARDS). Transmembrane RAGE is expressed by alveolar epithelial type I cells, and the variable domain of this receptor binds various ligands including high-mobility group box 1 (HMGB1). Binding of ligands to the ectodomain of RAGE can promote 1) lung inflammation in the acute-exudative phase of ARDS by activating NF-κB to increase the expression of proinflammatory genes and 2) fibroproliferative responses of the lung in the subacute phase of ARDS by increasing lung production of profibrotic growth factors including transforming growth factor-β (TGF-β) and platelet-derived growth factor (PDGF) and inducing epithelial-mesenchymal transition (EMT). MMP-3 and -13-mediated shedding of RAGE ectodomain releases soluble RAGE (sRAGE) forms. The latter may block pathological processes in the lungs of ARDS patients by acting as decoy receptors that bind RAGE ligands to prevent them from inducing intracellular signaling via the transmembrane form of RAGE.