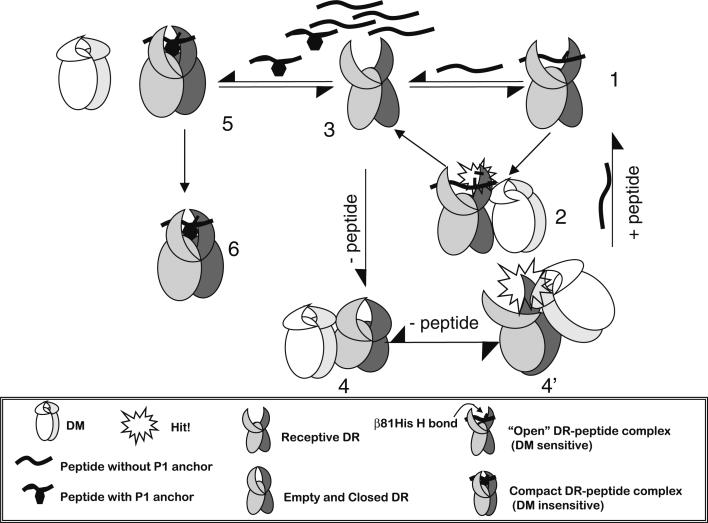
Fig. 1.
A model for heterogeneity of MHC class II conformations as the basis for DM recognition its effector function. A newly synthesized MHC class II, or DR1, molecule, occupied here by a peptide that does not fill Pocket 1, is in a ‘floppy’ or ‘open,’ DM-sensitive conformation (1). DM can interact transiently with the molecule by using the proposed ‘hit-and-run’ mechanism [39, 46] and can induce local conformational changes that lead to break in the b81His hydrogen bond between the peptide and DR1, resulting in the release of peptide (2). This generates a peptide-receptive DR1 (3), upon which several events might follow. The molecule can bind another peptide that is similar to the one described above and can then go through another round of DM-mediated dissociation (steps 1 and 2). Alternatively, in the absence of peptide, the DR1 molecule might close and become inactive over time under physiological conditions (4). The empty DR1 might now be rescued by a DM ‘hit’ to generate the peptide-receptive form again (4’). Finally, if DR1 binds a peptide that fills P1, the molecule then changes to a tight, DM-insensitive conformation (5). DM cannot interact productively with this complex, and the DR1 bound to peptide is exported to the cell surface (6)