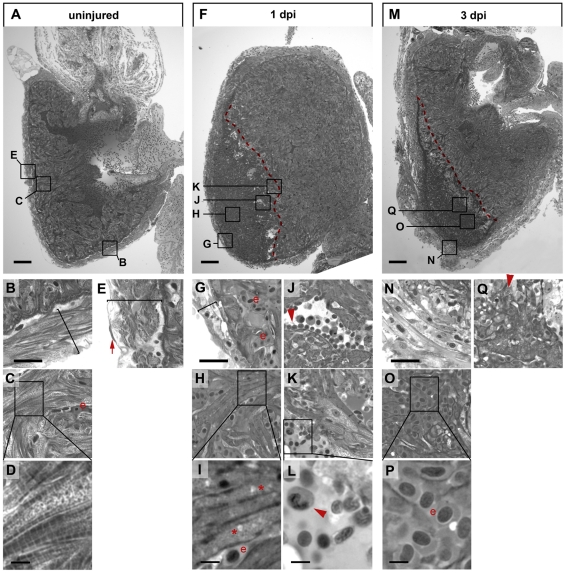
Figure 2. Cryoinjury causes cardiomyocyte death, inflammatory response and massive changes in tissue morphology.
Semi-thin histological sections of an uninjured heart (A–E) and cryolesioned hearts at 1 dpi (F–L) and 3 dpi (M–Q) stained with Tuloidin blue are shown. Wound edges are indicated with red dashed lines (F, M). Note striated cardiomyocytes in the compact external (B) and in the trabeculated internal (C, D) myocardium in the uninjured heart and the single layer of epicardial epithelium covering the myocardium (red arrow in E). (F–L) At 1 dpi, the thickness of the external myocardial layer is reduced (compare brackets in B and G) in the lesioned area, and most cardiomyocytes have lost striations suggesting myofibril disassembly (G, H, I). Also note appearance of large vacuolar structures (asterisk in I) indicative of cell death. Leukocytes, largely heterophil granulocytes, delineate the wound edges (J, K, L; see red arrowheads). (M–Q) At 3 dpi the external ventricular layer displays a loosened morphology but is devoid of cardiomyocytes (N). At 3 dpi the lesioned area is largely filled with erythrocytes (“e” in O, P). Cellular debris in the wound area is often associated with granulocytes (red arrowhead in Q). For each time point n = 3 hearts. Scale bars in A, F and M are 100 µm. Scale bars in B, G and N are 25 µm. Scale bars in D, I, L and P are 5 µm.