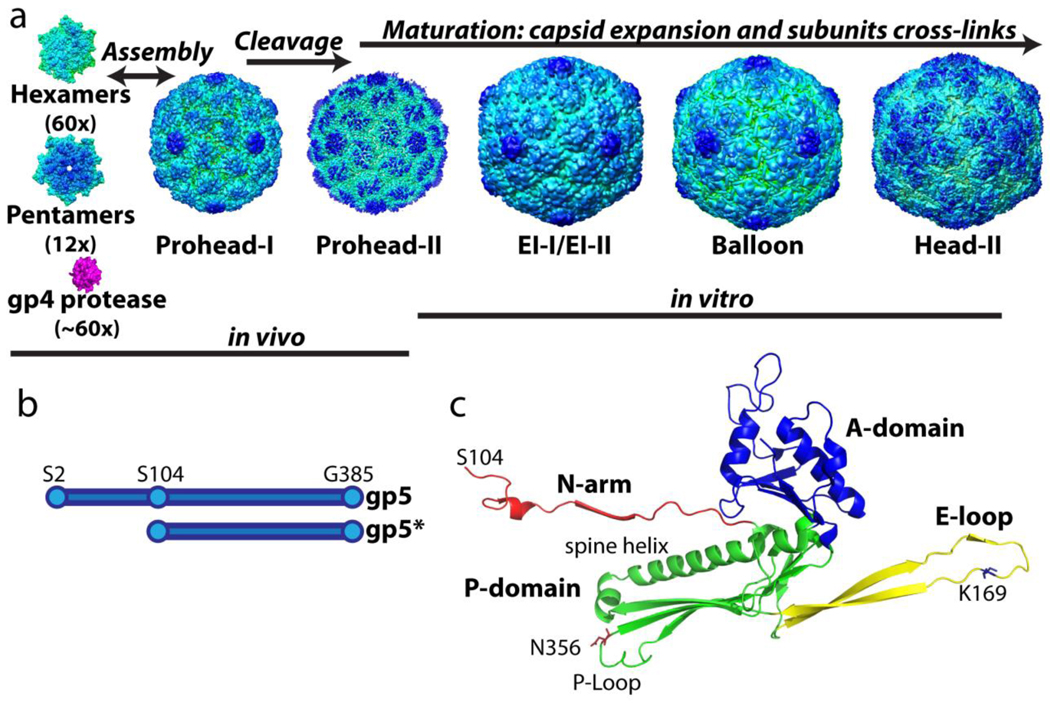
Figure 1.
The assembly and maturation pathway of HK97 virus-like-particles. (A) The hexamers and pentamers, composed of full length gp5 (residue 2–385 shown in B,) co-assemble with the viral protease gp4 to form Prohead-I. Once the assembly is complete, the protease cleaves off the delta domain (residue 2–103) followed by auto-proteolysis to form Prohead-II composed of gp5*. Capsid assembly and delta domain proteolysis take place in the E. coli expression system. When Prohead-II is purified by previously described methods, it can be matured in vitro under a variety of conditions8; 37. The final mature capsid, Head-II, contains catenated networks (“chain-mail”) formed by isopeptide covalent bonds between subunits. (C) The crystal structure of the Head-II capsid subunit. The two domains and two extensions are color coded. Cross-links join lysine 169 on the E-loop of one subunit to asparagine 356 on the P-domain of the neighboring subunit. The delta domain, which is absent in Prohead-II and Head-II, is connected to the N-arm in capsomers and in Prohead-I.