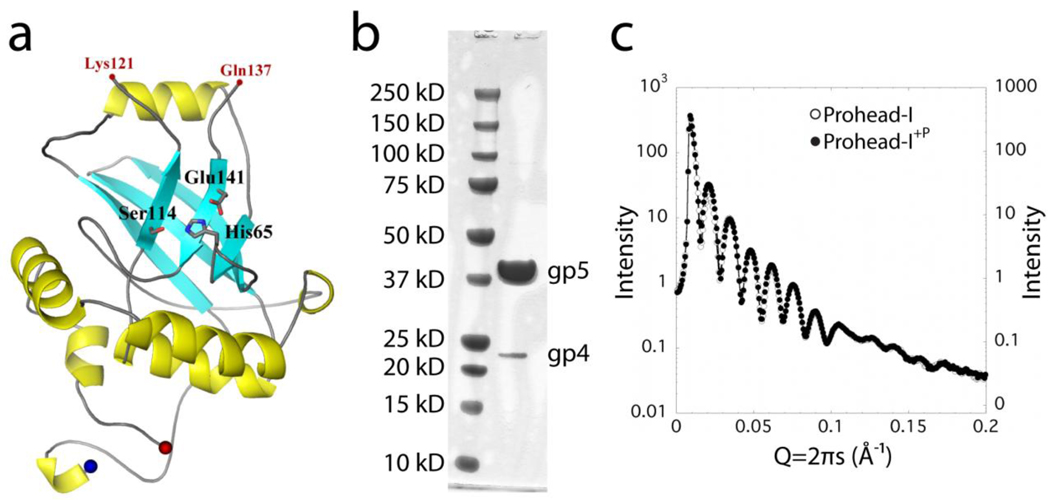
Figure 2.
Prohead-I+P encapsidates enzymatically inactive gp4 protease. (A) The proposed model of gp4 protease generated using Herpes protease homology. The active site of the serine protease is shown in stick representation. (B) SDS-PAGE of Prohead-I with inactive protease. Prohead-I gp5 is at 42 kDa and the gp4 protease is at 24 kDa. Gp4 is incorporated into the Prohead-I capsid. (C) Overlapping curves from Prohead-I with and without protease in their SAXS patterns indicate that both forms have virtually identical particle shape and overall structure at this resolution.