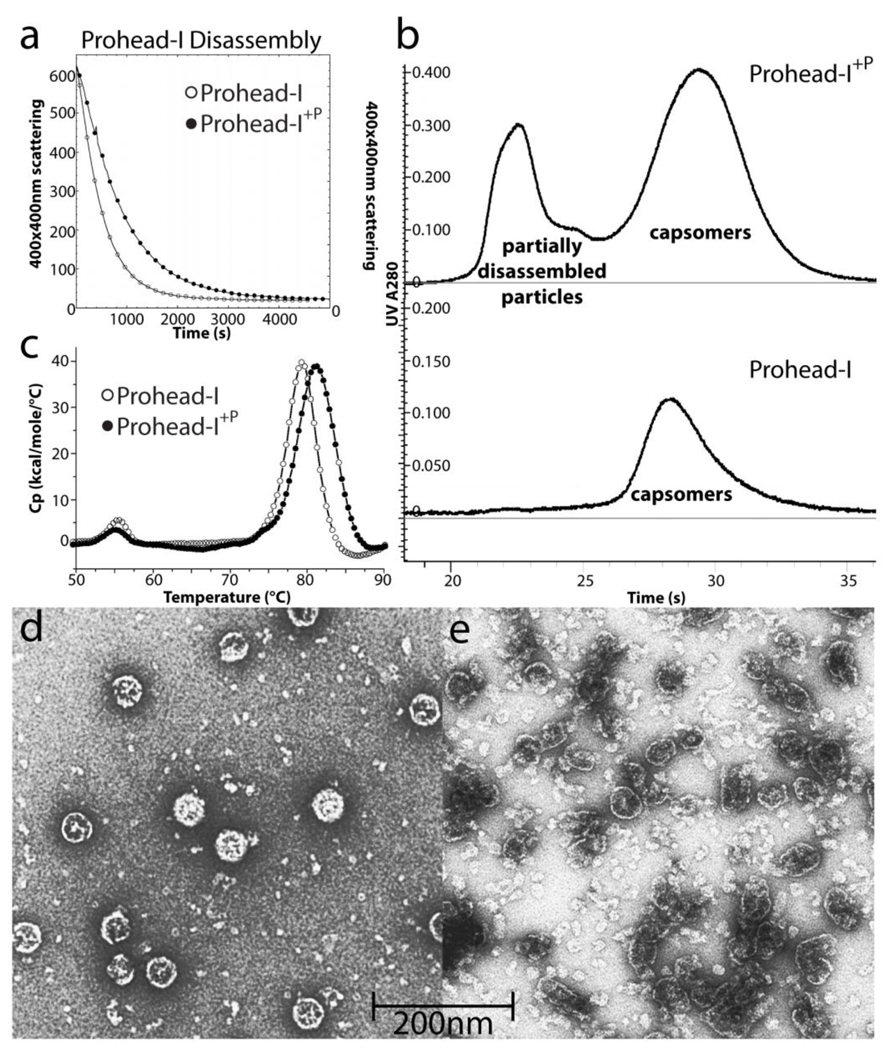
Figure 3.
Biochemical comparisons of Prohead-I with and without protease. (A) Disassembly kinetics of Prohead-I and Prohead-I+P monitored by light scattering at 400nm. By incorporating inactive protease, Prohead-I capsid disassociated significantly slower than without protease, indicating capsid stabilization by protease-delta domain interactions. (B) Both forms of Prohead-I were loaded into Superdex 200 size exclusion column after disassembly overnight. Prohead-I+P remains partially disassembled while Prohead-I was completely disassociated after overnight in disassembly buffer. (C) The thermogram of both forms of Prohead-I analyzed by Differential Scanning Calorimetry. Prohead-I undergoes a major thermal event at 79°C while Prohead-I+P undergoes a similar transition at 82°C. The recorded thermal event corresponds to particle disassembly and protein denaturation since there is no additional peak up to 110°C (not shown). Three degrees of thermal stabilization is contributed by interacting with the protease. (D) Negative stain electron microscopy of intact Prohead-I+P and (E) partially assembled particles eluted from the size exclusion column show that subjecting these protease-containing particles to conditions that completely dissociate wild type Prohead-I causes severe damage to the particles but does not fully disassociate them.