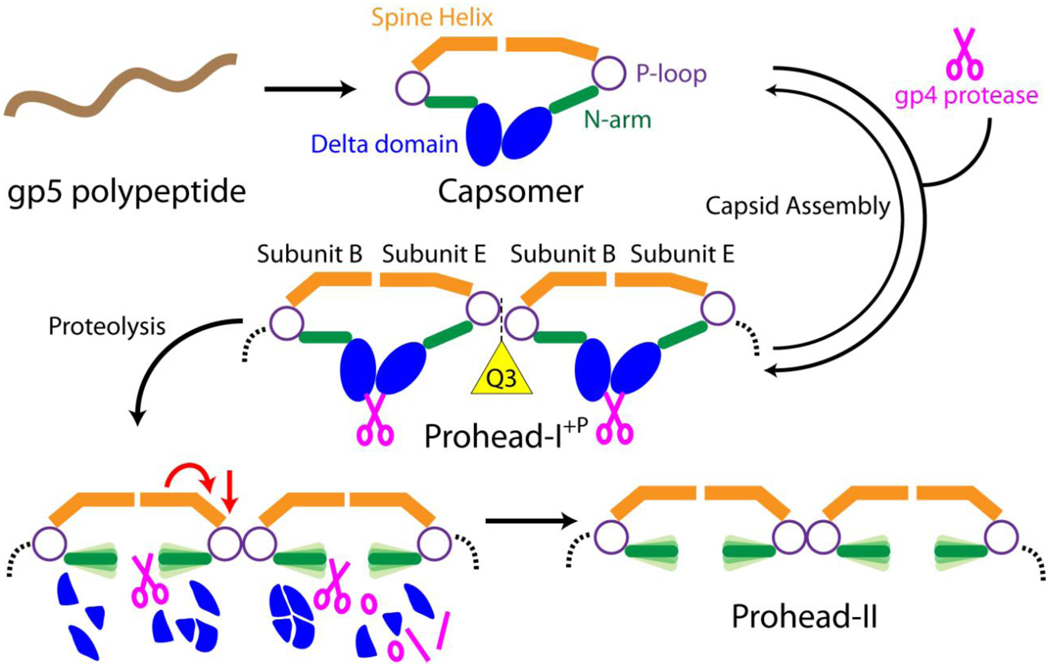
Figure 7.
A schematic illustration that depicts subunit interactions and transitions during maturation to Prohead-II. The gp5 polypeptide is expressed and immediately folds into capsomers in E. coli. Incorporating the gp4 protease, the delta domains promote inter-capsomer interactions to drive formation of Prohead-I+P. In this particle, the delta domain serves as a lock to prevent maturation by demobilizing the N-arms. As a result, the Class 2 quasi 3-fold contacts, composed of subunits B, E (shown in the figure) and C (not shown in the figure), are open whereas other trimer contacts are sealed. Protease removes the delta domain, allowing the N-arms to gain flexibility, and the spine helix in subunit E undergoes additional bending to completely close the quasi 3-fold contact. The transformed particle, Prohead-II, is a meta-stable intermediate that is energetically positioned for spontaneous maturation when electrostatically triggered.