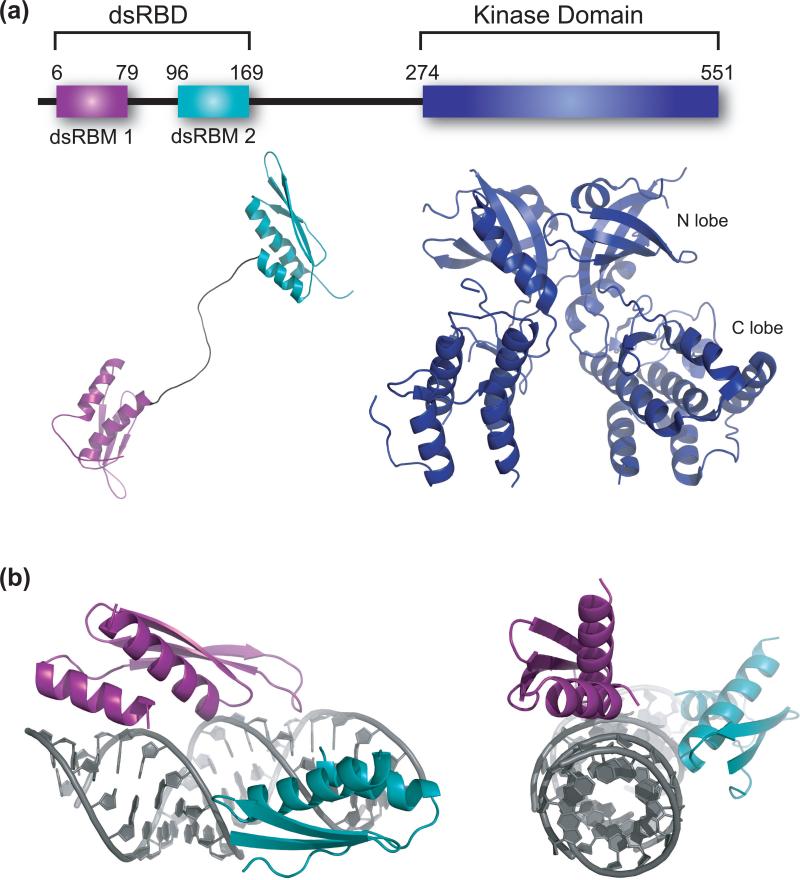
Figure 2.
Structural biology of PKR. (a) Structures of the two domains of PKR. PKR is a 551 amino acid protein that contains an N-terminal dsRNA binding domain (dsRBD) that is comprised of two dsRNA binding motifs (dsRBMs) spaced by a flexible 20 amino acid linker depicted below the dsRBMs, and a C-terminal kinase domain with small and large N- and C-terminal domains. Available are an NMR structure of the dsRBD (pdbid 1QU6) and a crystal structure of the kinase domain complexed with eIF2α substrate (eIF2α omitted here). The kinase crystallizes as a dimer (pdbid 2A1A). (b) Structure of a dsRBM from X. laevis rbpa bound to dsRNA (pdbid 1DI2) [16]. The dsRNA is 10 bp in length, and two helices are shown stacked end-to-end. Each dsRBM occupies one face of the dsRNA, and packing occurs along different faces of the dsRNA. Shown are side-on and end-on views.