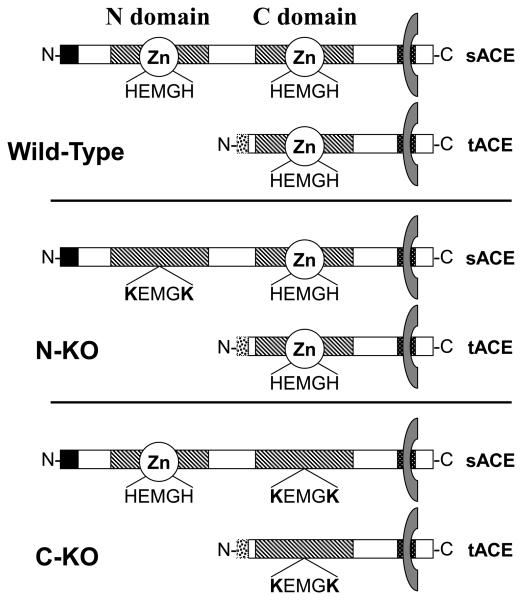
Figure 1.
Schematic representation of the WT, N-KO and C-KO ACE enzyme. The somatic isoform of ACE (sACE) is composed of two homologous regions termed N- and C- domains (hatched regions). Each domain contains the zinc-binding catalytic motif HEMGH. These domains are anchored to the plasma membrane by a hydrophobic region located at the carboxy-terminus extremity of the protein. In the N-KO strain the catalytic site of the N-domain was mutated to KEMGK abolishing its ability to bind zinc and rendering it inactive. In the C-KO strain, similar mutations inactivated the C-domain. The testis isoform (tACE), exclusively expressed in male germ cells, lacks the N-domain of sACE and has only one catalytic domain.