Table 3.
One-Pot Palladium-Catalyzed Cross-Coupling Reactions
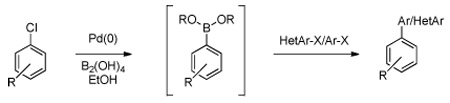 | ||||
|---|---|---|---|---|
| entry | first chloride | second chloride | product | isolated yield (%) |
| 1 | 85 | |||
| 2 | 90 | |||
| 3 | 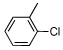 |
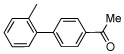 |
63 | |
| 4 | 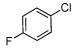 |
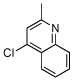 |
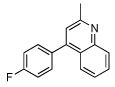 |
60 |
| 5 | 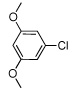 |
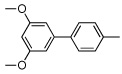 |
55 | |
General conditions: 2.5 mol % of 2, 5 mol % of X-Phos, 2.5 mol % of NaOt-Bu, 3 equiv of KOAc, 3 equiv of 1, EtOH (0.1 M), 80 °C, 2 h. 1 equiv of the second aryl or heteroaryl chloride, 3 equiv of 1.8 M K2CO3, 80 °C, 15 h.
