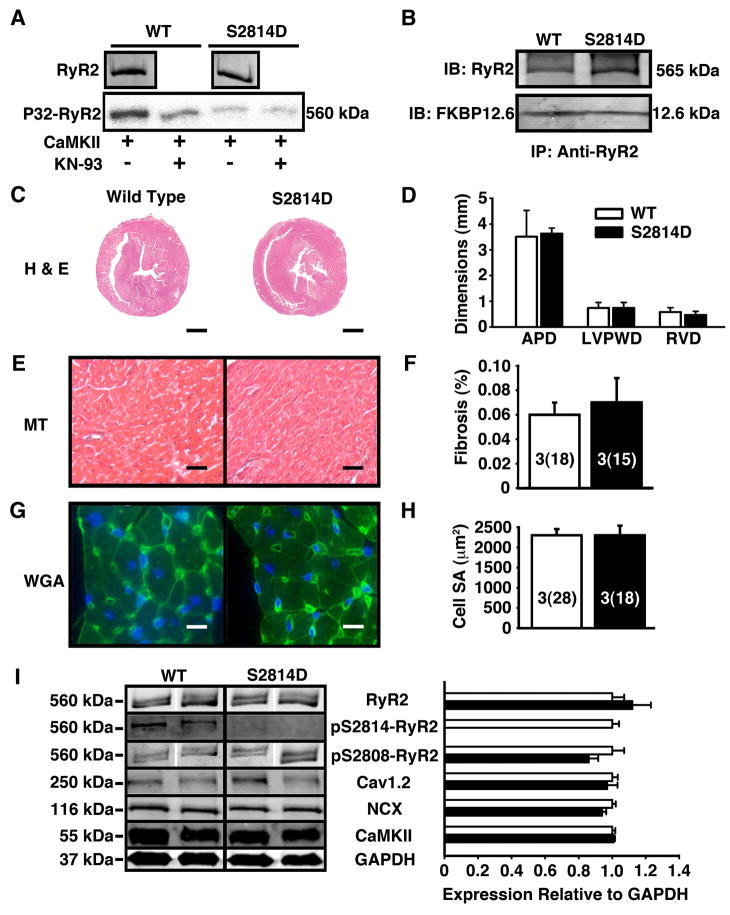
Figure 1. Baseline molecular and structural characteristics of S2814D mice.
(A) The S2814D mutation prevents CaMKII mediated phosphorylation of RyR2. Equal amounts of RyR2 protein were immunoprecipitated from WT and S2814D hearts and phosphorylated with CaMKII in the presence or absence of KN93. Shown is a representative example of an experiment repeated twice. (B) Co-immunoprecipitation showing that the binding of FKBP12.6 to RyR2 was not altered in S2814D mice. (C) Representative histological sections of 3 month-old mouse hearts stained with hematoxylin and eosin (H&E). Scale bar lines represent 1 mm (D) Cardiac wall dimensions assessed from H&E histological sections. Left-ventricular (LV) anterior-posterior diameter (APD), LV posterior wall diameter (LVPWD) and right ventricular (RV) wall diameter (RVD) were similar among WT and S2814D hearts. (E) Masson’s Trichrome for fibrosis of histological sections of 3 month-old mouse hearts. Scale bar lines represent 30 μm. (F) Percent fibrosis quantified from Masson’s Trichrome stain. (G) Representative wheat germ agglutinin fluorescence stains (WGA) of mouse cardiomyocytes. Scale bars represent 10 μm. (H) Cardiomyocyte surface area calculated from WGA stains. (I) Western blot and phosphorylation assays showing baseline protein expression and phosphorylation of RyR2 at baseline. On Western blot, expression of the cardiac ryanodine receptor (RyR2), the L-type Ca2+ channel (Cav1.2), Na+/Ca2+-exchanger (NCX), and Ca2+/calmodulin kinase II (CaMKII) were not statistically different between WT and S2814D mouse hearts at rest. GAPDH was used as a loading control for Western Blots. Number of animals (number of cells) is indicated in bar graphs.