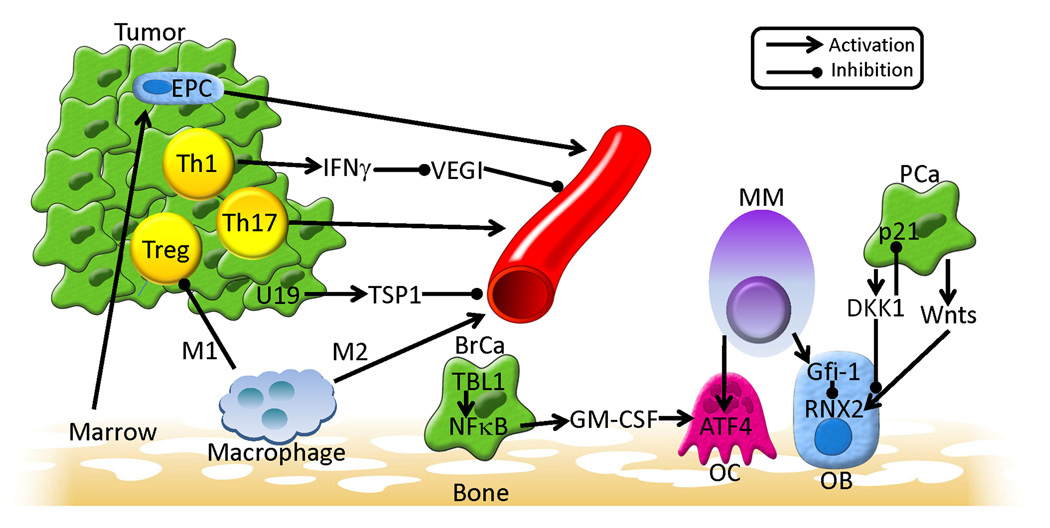
Figure 1. Summary of selected key points from The First Tianjin, China Forum on Tumor Microenvironment.
A variety of host and tumor interactions can promote angiogenesis. (1) Marrow-derived endothelial precursor cells (EPC) can localize to tumor where they can participate in vascular formation; (2) Intratumoral Th1 cells, which produce interferon-gamma (IFNγ) can inhibit vascular endothelial growth cell inhibitor (VEGI), which itself is an inhibitor of angiogenesis, thus resulting in an overall pro-angiogenic effect; (3) Both intratumoral Th17 cells and M2-differentiated macrophages promote angiogenesis through cytokine secretion; and (4) tumor-associated decrease of upregulated gene 19 (U19) expression results in decreased thrombospondin-1 (TSP) expression, which itself inhibits angiogenesis, resulting in an overall pro-angiogenic effect. Macrophages can also differentiate along an M1 pathway, resulting in inhibition of T regulatory (Treg) cells which will result in derepressing a variety of T cell responses that can promote tumor growth and angiogenesis. In addition to angiogenic effects, there were multiple interactions identified that result in impacting the bone microenvironment. In breast cancer (BrCa) it was show that transducin-β-like protein 1 (TBL1) was required for induction of NFκB activity, which resulted in production of granulocyte monocyte-colony stimulating factor (GM-CSF) and osteoclast (OC) production. Multiple myeloma (MM) was also shown to simulate OC differentiation through activation of activating transcription factor 4 (ATF4). In addition to its impact on OCs, MM also promotes overall bone loss through inhibition of osteoblasts (OB) by upregulating Gfi-1 expression, which in turn inhibits the RNX2 transcription factor that is required for osteoblastogenesis. In contrast, prostate cancer (PCa) was shown to promote osteoblast activity through downregulation of dikkopf-1 (DKK1), which allows for Wnts to promote osteoblastic activity. DKK1 also was shown to promote the cell cycle through activation inhibition of the cell cycle inhibitor p21, which overall promotes tumor growth.