Figure 7.
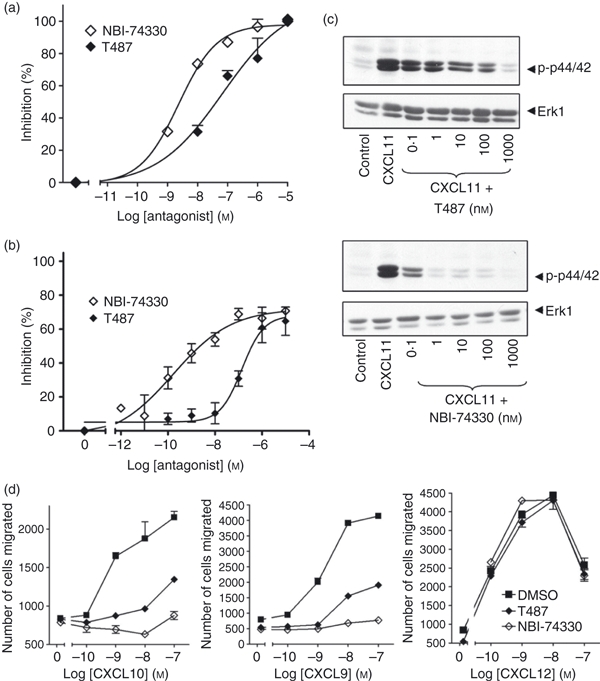
CXCR3 antagonists inhibit CXCL11-mediated responses in human T cells. Staphylococcus enterotoxin B (SEB)/interleukin-2 (IL-2) activated T lymphocytes (3·2 × 106 cells/ml; day 9–12 post-activation) were incubated for 30 min with the CXCR3 antagonists T487 or NBI-74330 at concentrations indicated (a, b, c) or 1 μm and 100 nm, respectively (d). Cells were then used in migration (a,d), biochemical (c) or receptor surface expression assays (b). For migration assays, cells placed on the upper membrane of a 96-well chemotaxis plate above a lower chamber containing 1 nm CXCL11 (a) or concentrations indicated (d). Cell migration across a 5-μm pore size membrane was determined as described in the Materials and methods. For receptor surface expression assays (b), T cells were stimulated with 30 nm of CXCL11 for 5 min. Agonist was then washed off and cells were incubated with anti-CXCR3 antibody or isotype control at 4° followed by analysis FACSCanto™ flow cytometer as described in the Materials and methods. Data (a, b, d) represent mean ± SEM of at least three independent experiments using cells from different donors. (c) Aliquots of activated T cells (1 × 106 cells/500 μl) were left untreated or stimulated at 37° with 1 nm CXCL11 for 2 min in the absence or presence of 30 min pre-treatment with T487 or NB-74330 at the concentrations indicated. Cell lysates were resolved by SDS–PAGE, transferred to nitrocellulose membranes, and immunoblotted with a phospho-specific Erk or Akt antibody with affinity for the active Ser473-phosphorylated form of Akt and proteins were visualized with enhanced chemiluminescence. The blots were stripped and reprobed with anti-Erk-1 antibody to verify equal loading and efficiency of protein transfer (lower panel). The data are derived from a single experiment representative of at least three others using cells from different donors.
