Figure 1.
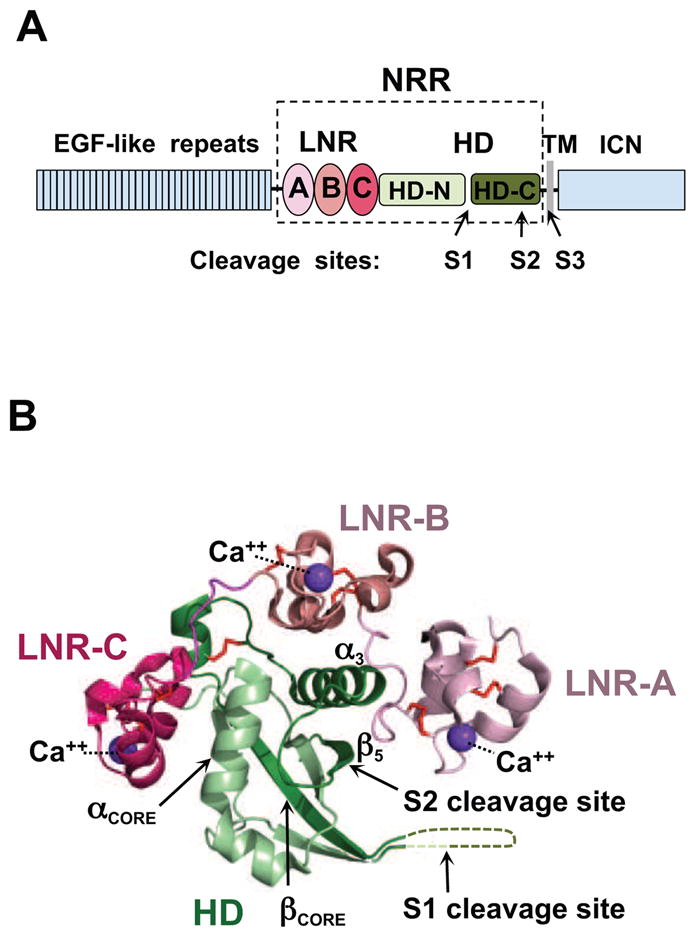
Domain organization and overview of the Notch1 NRR structure. (A) Domain organization of Notch1. The extracellular portion of the receptor consists of 36 EGF-like repeats responsible for ligand binding (blue), and the negative regulatory region (NRR, boxed) that maintains proteolytic resistance in the absence of ligands. The NRR encompasses three LIN12/Notch repeats (LNR-A, LNR-B, and LNR-C, colored in different shades of red), and the heterodimerization domain (HD, green), divided at S1 by a furin-like protease during maturation. Ligand binding to the extracellular portion of Notch triggers metalloprotease cleavage at site S2. The resulting truncated transmembrane subunit of the receptor is a substrate for cleavage at S3 by gamma-secretase, which releases the intracellular part of Notch (ICN, blue) from the membrane. (B) Ribbon diagram of the Notch1 NRR in its autoinhibited conformation (PDB ID code 3IO8). The three LNR modules are shown in different shades of pink, and the HD domain is in green. Disulfide bonds are orange, and the three calcium ions coordinated by the LNR modules are purple. Key secondary structural elements and the S1 and S2 cleavage sites are also indicated. See also Figure S1.
