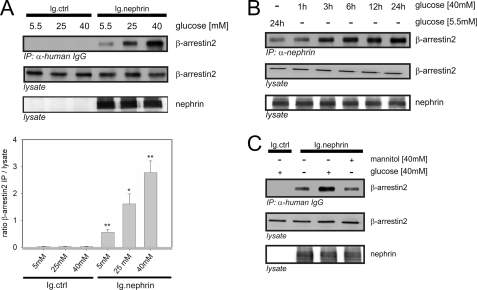
FIGURE 1.
High glucose concentrations increase the binding of β-arrestin2 to nephrin. Coimmunoprecipitation experiments were conducted in HEK293T cells and analyzed by Western blotting. FLAG-tagged β-arrestin2 (β-arrestin2), Ig-tagged nephrin C terminus (Ig.nephrin), and Ig tag as the negative control (Ig.ctrl) were overexpressed as indicated. Staining of β-arrestin2 and nephrin in the lysate served as the internal loading control. A, impact of rising glucose concentration. HEK293T cells were incubated in the medium with increasing concentrations of glucose (5.5, 25, or 40 mm) for 24 h. After the indicated time points, coimmunoprecipitation with Ig.nephrin or Ig.ctrl was performed. Interaction was determined by staining of β-arrestin2. The results of three independent experiments were quantified by densitometry and graphed as the ratio of the β-arrestin2 immunoprecipitation (IP) signal intensity to the lysate signal intensity (ratio β-arrestin2 IP/lysate). The data are the means ± S.E. *, p < 0.05; **, p < 0.01 (Student's t test). B, time course. The cells were incubated with 5.5 mm glucose for 24 h or 40 mm glucose for 1–24 h. Then coimmunoprecipitation with Ig.nephrin or Ig.ctrl was performed. The degree of interaction was determined by staining of β-arrestin2. C, osmotic control. The cells were incubated with 5.5 or 40 mm glucose or 40 mm mannitol for 24 h. Then coimmunoprecipitation with Ig.nephrin or Ig.ctrl was performed. The degree of interaction was determined by staining of β-arrestin2.