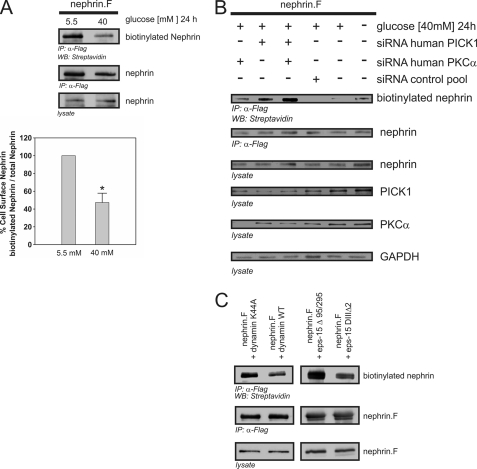
FIGURE 7.
High glucose-induced nephrin endocytosis depends on PKCα and PICK1. A–C, surface abundance of nephrin measured in biotinylation assays. FLAG-tagged full-length nephrin (nephrin.F), functional dynamin (dynamin WT), and epsin (eps-15Δ95/295), and the according dominant negative mutants (dynamin K44A and eps-15 DIIIΔ2) were transiently overexpressed in HEK293T cells. A, impact of high glucose concentrations on nephrin surface abundance. After immunoprecipitation, surface nephrin was determined by detecting the biotinylated fraction. Staining of nephrin in the lysate and immunoprecipitation served as the internal control. The results were quantified by densitometry and graphed as the ratio of the PKCα immunoprecipitation (IP) signal intensity to the lysate signal intensity (ratio PKCα IP/lysate). The data are the means ± S.E. (n = 4). *, p < 0.001 (Student's t test). B, impact of cellular knockdown of PICK1 and PKCα on nephrin surface abundance. Specific siRNA was used to deplete HEK293T cells of endogenous PKCα and/or PICK1. The cells were maintained in 5.5 or 40 mm glucose medium for 24 h. GAPDH was used as the internal loading control. First column, PKCα siRNA; second column, PICK1 siRNA; third column, PKCα and PICK1 siRNA. For the controls: fourth column, scrambled control siRNA; fifth column, no siRNA (40 mm glucose); sixth column, no siRNA, transfection lipid only (5.5 mm glucose). Biotinylated surface nephrin was stained with streptavidin (first row). Total expression levels of nephrin are shown in the second row (immunoprecipitation, IP) and the third row (lysate). The expression levels of PICK1, PKCα, and GAPDH are shown in the fourth, fifth, and sixth rows, respectively. C, nephrin endocytosis depends on dynamin and clathrin. FLAG-tagged full-length nephrin (nephrin.F), dynamin (wild-type and dominant negative mutant K44A), and epsin (eps-15Δ95/295 and dominant negative mutant eps-15 DIIIΔ2) were transiently overexpressed in HEK293T cells. After immunoprecipitation, surface nephrin was determined by detecting the biotinylated fraction. Staining of nephrin in the immunoprecipitation and lysate served as the internal loading control.