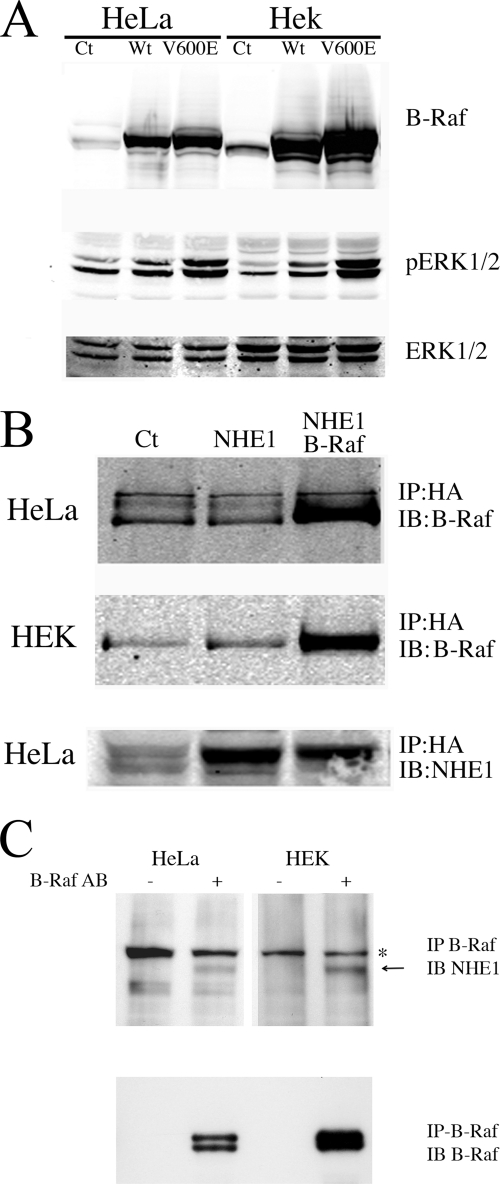
FIGURE 1.
Characterization of B-Raf expression and its interaction with NHE1 in HeLa and HEK cells. HeLa or HEK cells were transiently transfected with wild type (Wt) B-Raf or V600E mutant B-Raf plasmid (V600E) for 24 h, and the cell lysates were prepared and run for Western blotting. Ct indicates mock-transfected cells. A, upper panel, Western blot with anti B-Raf antibody. Middle panel, Western blot with anti-phospho-ERK1/2 antibody. Lower panel, Western blot with anti ERK1/2 antibody. Results are typical of three experiments. B, co-immunoprecipitation of (wild type) B-Raf and NHE1. HEK or HeLa cells were transiently transfected with an expression plasmid for NHE1 (NHE1) and for wild type B-Raf where indicated. NHE1 was then immunoprecipitated (IP) using antibody against the NHE1-HA tag as described under “Experimental Procedures.” Immunoblotting (IB) was done with anti-B-Raf antibody or with anti NHE1 protein antibody as indicated. C, immunoprecipitation of endogenous NHE1 and B-Raf. Confluent HeLa or HEK293 cells were cross-linked in the presence of dithiobis(succinimidylpropionate), and cell lysates were prepared as described under “Experimental Procedures.” B-Raf was immunoprecipitated (IP) using antibody against B-Raf where indicated. The co-IP complex was solubilized with SDS-PAGE sample buffer containing 10 mm DTT for 30 min at 37 °C. After SDS-PAGE, proteins were transferred onto nitrocellulose for immunoblotting (IB), which was done with anti-NHE1 antibody (Millipore, Temecula, CA) or with anti-B-Raf antibody as indicated. Arrow denotes location of NHE1 protein. * indicates nonspecific protein present in all samples. Results are typical of at least three experiments.