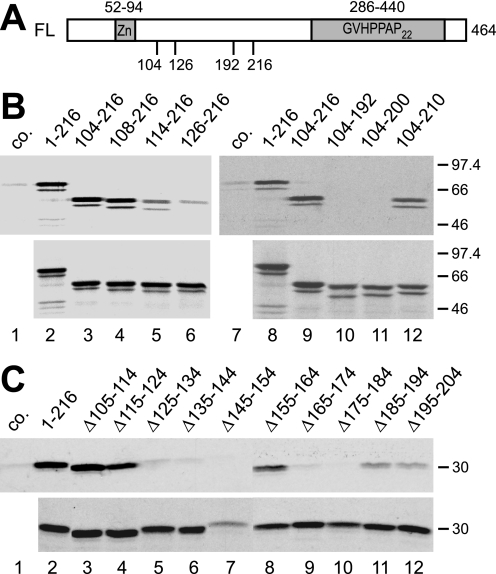
FIGURE 2.
Amino acids 108–216 of SF3a66 are sufficient for the interaction with SF3a120. A, schematic representation of the SF3a66 domain structure. The SF3a66 sequence was taken from accession no. Q15428. The zinc finger domain (Zn) and Pro-rich heptad repeats (GVHPPAP22) are indicated. Numbers below the scheme refer to the maximal and minimal extent of SF3a66 deletion mutants. B, GST pulldown of N- and C-terminal SF3a66 deletion mutants. In vitro-translated SF3a66 proteins indicated above the figure were incubated with GST-3a120/269–295 bound to glutathione-agarose (lanes 2–6 and 8–12). As a control, 3a66/1–216 was incubated with GST-coupled beads (lanes 1 and 7, co.). Bound proteins (top) and 20% of the input proteins (bottom) were separated by 10% SDS-PAGE and visualized by autoradiography. C, GST pulldown of SF3a66 proteins with internal 10-amino acid deletions. The experiment was performed as in B, except that a 12% SDS gel was used. Lane 1, 3a66/1–216 was incubated with GST (co.) lanes 2–13, proteins shown above the figure were incubated with GST-3a120/269–295.