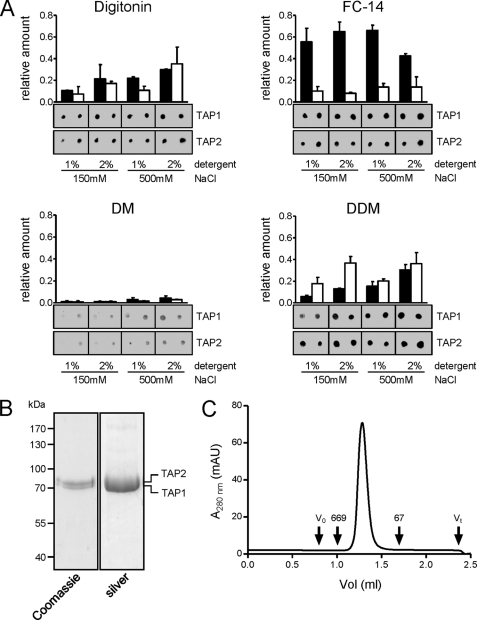
FIGURE 3.
Purification of the TAP complex. A, purification screen. Crude membranes (10 mg of protein) were solubilized with 1% or 2% (w/v) of detergent at either low-salt (150 mm) or high-salt (500 mm) concentrations. TAP was purified by metal affinity chromatography. Recovery was analyzed by immunoblotting using α-TAP1 and α-TAP2 antibodies. All conditions were analyzed in independent duplicates. Representative results for digitonin, Fos-Choline-14 (FC-14), DM, and DDM are shown; all other results are summarized in supplemental Fig. S1. Error bars show S.D. B, TAP purification. Crude membranes were solubilized in 2% digitonin and purified by metal affinity chromatography. Purified protein was analyzed by SDS-PAGE (10%). C, monodispersity of the purified TAP complex was verified by size exclusion chromatography (Superose 6 PC 3.2/30). V0, void volume; Vt, total volume; the elution volumes of thyroglobulin (669 kDa) and bovine serum albumin (67 kDa) are indicated. mAU, milliabsorbance units.