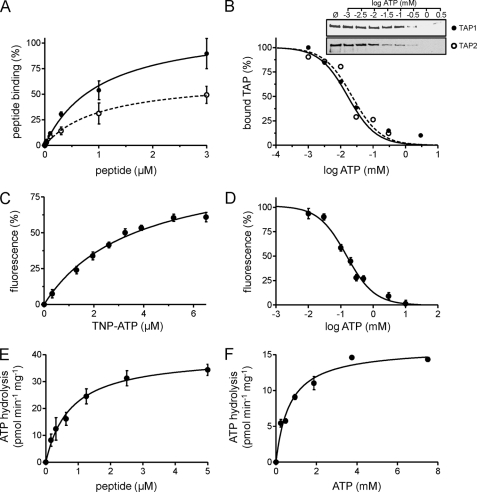
FIGURE 4.
Activity of purified TAP. A, peptide binding. TAP (10 μg) purified from P. pastoris (filled circles) or insect cells (open circles) was incubated with increasing amounts of 125I-labeled peptide RRYQKSTEL in the absence or presence of a 200-fold excess of unlabeled peptide. Data were fitted to a Langmuir (1:1) binding isotherm. B, ATP binding. 3.5 μg of purified TAP was bound to ATP-agarose in the presence of increasing concentrations of ATP. TAP bound to ATP-agarose was analyzed by SDS-PAGE and immunoblotting. Intensities were quantified and fitted to a dose-response curve. C, TNP-ATP binding. Purified TAP (0.1 μm) was incubated with increasing amounts of TNP-ATP. The fluorescence intensity at λex/em 410/541 nm was plotted against the respective TNP-ATP concentration, and data were fitted to a Langmuir (1:1) binding isotherm. D, competitive binding assay. Purified TAP (10 μg) was incubated with 6.5 μm TNP-ATP in the presence of increasing concentrations of ATP. Fluorescence intensity at λex/em 410/541 nm was plotted against ATP concentration. E and F, peptide stimulated ATP hydrolysis of TAP. Purified TAP (6 μg) was measured with increasing concentrations of RRYQKSTEL (E) or ATP (F) and fitted to the Michaelis-Menten equation. Each data point represents the mean of triplicate measurements. Error bars show S.D.