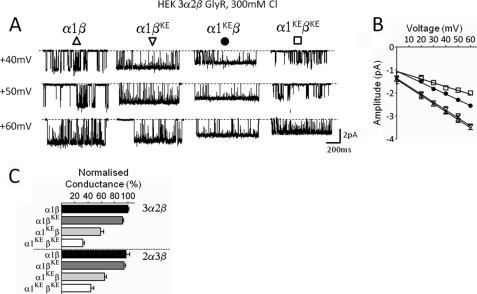
FIGURE 4.
In the α1β Gly channels the pattern of effect of mutations in the different subunits is not altered by increasing permeant ion concentration. Representative traces of outward currents recorded in cell-attached patches at different voltages (display fc = 1 kHz) in 300 mm chloride (solution S4 in Table 1). A shows example of traces recorded at positive voltages for different combinations of wild-type and mutated subunits for the heteromeric α1β Gly channels. B, outward current-voltage relationships for the wild-type and mutant heteromeric α1β receptor: α1β (Δ), α1βKE (▿), α1KEβ (●), α1KEβKE (□). C, summary of outward slope conductance values for GlyRs with 3α:2β (HEK, upper panel) and 2α:3β (expressed as concatenated subunits in oocytes, lower panel) stoichiometries. Values are normalized to the conductance value of the wild type. Note that when both subunits are mutated the conductance decreases more markedly in the 3α form (32.2% of the wild type) rather than in the 2α form of the receptor (45.6% of the wild type).