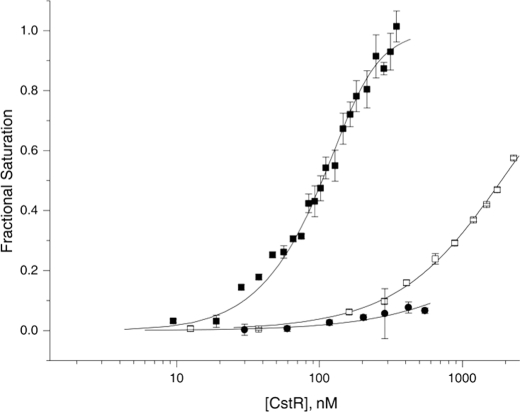
FIGURE 6.
The DNA binding activity of CstR is negatively regulated by covalent cysteine disulfide and trisulfide derivatization by sodium sulfite. Unmodified fully reduced (solid squares, ■), sulfite-treated (open squares, □), and MMTS-reacted (solid circles, ●) CstRs were titrated into a solution of 10 nm Cst OP1 DNA and anisotropy (ri) was recorded in triplicate. Each data point represents the mean ± S.D. following each ith addition of protein, following normalization to the starting (ro) and ending (rcomplex) values of the anisotropy of the underivatized CstR complex. The solid line is a fit to a two-tetramer binding model, with the anisotropy of the 1:1 complex fixed to 0.5 × (rcomplex − ro). Fitted parameters are complied in Table 1. Conditions used were 10 mm Hepes, pH 7.0, 0.2 m NaCl, at 25.0 °C.