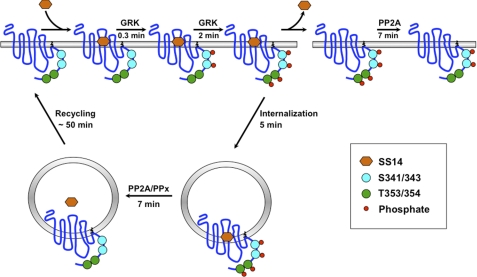
FIGURE 10.
Specificity in the phosphorylation and dephosphorylation of neighboring GRK sites in the sst2A receptor. Upon binding of SS14, the sst2A receptor is activated and becomes rapidly phosphorylated by GRKs first on Ser-341/343 and subsequently on Thr-353/454. In the continued presence of agonist, the phosphorylated receptor is internalized. However, if agonist is removed while the receptor is on the cell surface, Thr(P)-353/354 are rapidly dephosphorylated by an okadaic acid and fostriecin-sensitive phosphatase, shown as PP2A. In contrast, phosphorylated Ser-341/343 are resistant to dephosphorylation while the receptor is on the plasma membrane. If agonist is removed after the receptor has been internalized, the receptor is dephosphorylated at both Ser-341/343 and at Thr-353/354 by two different phosphatases. An okadaic acid/fostriecin-sensitive phosphatase (shown as PP2A) again dephosphorylates Thr-353/354 in internalized receptors. In contrast, Ser-341/343 is dephosphorylated by a phosphatase (PPx), which is resistant to okadaic acid and fostriecin as well as FK506 and thus may belong to the PP2C phosphatase families, for which no inhibitors have been identified. Dephosphorylated sst2A receptors are recycled back to the plasma membrane for another round of signaling.