FIGURE 9.
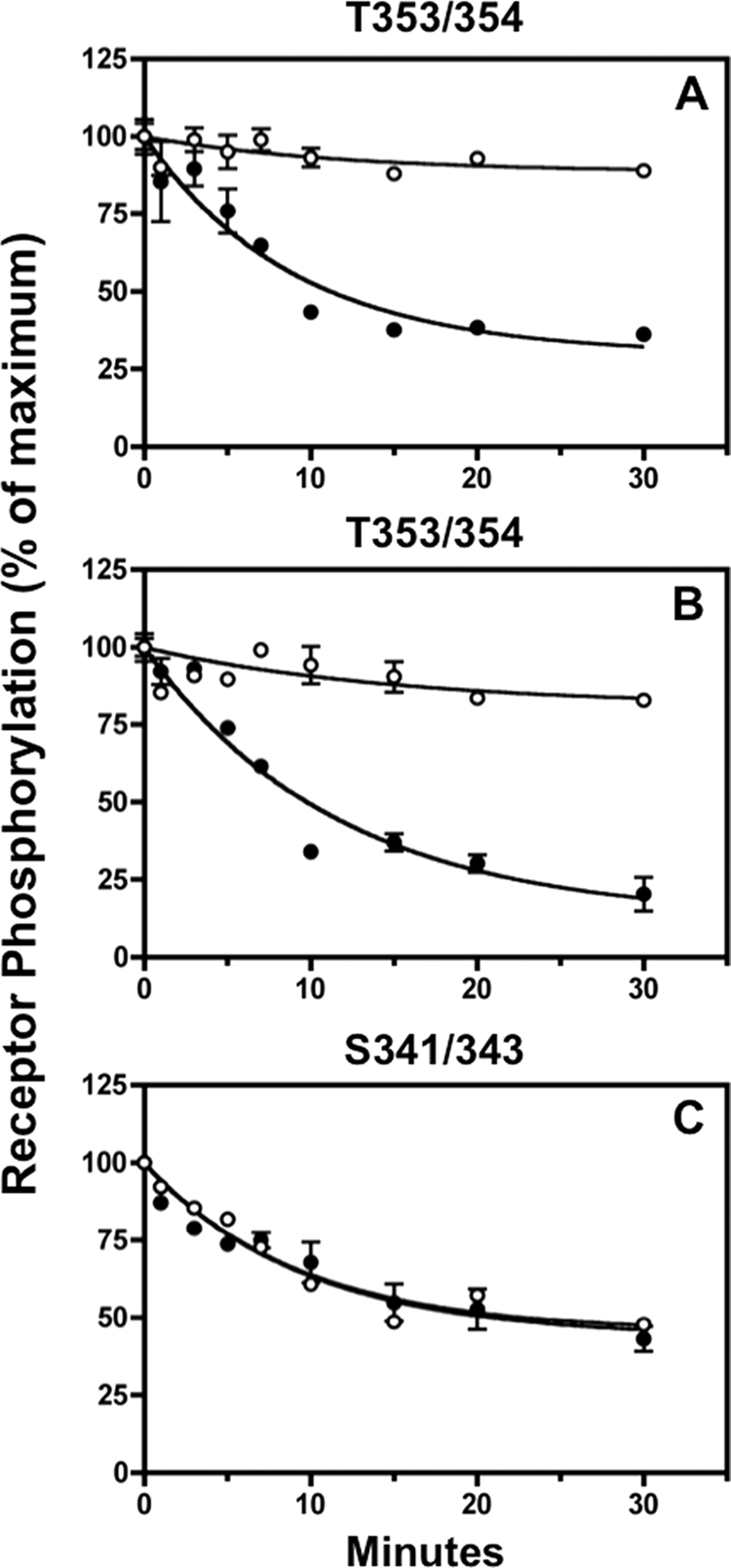
Fostriecin inhibits sst2A receptor dephosphorylation at Thr-353/354 but not at Ser-341/343. Panel A, CHO-sst2A cells were preincubated without (●) or with (○) 5 nm fostriecin for 15 min at 37 °C and then stimulated with 10 nm SS14 for either 2 min (panel A) or 15 min (panels B and C) in the continued absence or presence of the phosphatase inhibitor. Following a rapid wash to remove agonist, cells were subsequently incubated for the times shown in fresh 37 °C medium without SS14 in the continued absence or presence of fostriecin. Cells were then fixed, and the residual receptor phosphorylation was measured with the in-cell ELISA. The graphs show the results of replicate samples in a representative experiment. In multiple independent experiments, the half-time for dephosphorylation of Thr-353/354 in control cells was 6.9 ± 1.1 min (n = 2) after 2 min of agonist exposure (cell surface receptors) and 7.3 ± 0.1 min (n = 2) after 15 min of agonist exposure (internalized receptors). Fostriecin blocked Thr-353/354 dephosphorylation both at the cell surface and intracellularly. In contrast, Ser-341/343 dephosphorylation was unaffected by fostriecin; the half-time for Ser-341/343 dephosphorylation was 8.6 ± 0.7 min (n = 2) min and 7.00 ± 1.0 (n = 2) min in the absence and presence of fostriecin, respectively.
