FIGURE 3.
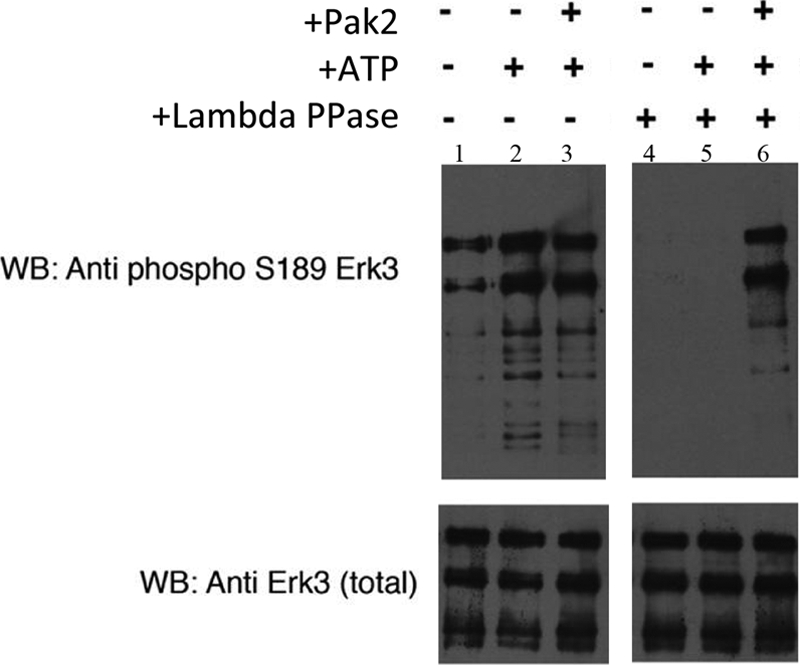
Pak2 phosphorylates serine 189 within the Erk3 activation loop in vitro. The Erk3 protein purified from insect cells is pre-phosphorylated on serine 189. Recombinant Erk3 protein (Fig. 2A, lane 8) was dialyzed into 1× phosphobuffer and subjected to an in vitro kinase assay using recombinant Pak2. Proteins were resolved by electrophoresis on an 8% SDS-polyacrylamide gel and subjected to Western analyses using phospho-Ser189 specific antisera (upper panel) and total Erk3 antibodies (lower panel) to ensure equivalent loading of Erk3. Lane 1, Erk3 protein in 1× phosphobuffer; lane 2, Erk3 protein after 30 min incubation with ATP; lane 3, Erk3 protein after 30 min of incubation with ATP and Pak2. Lanes 4–6 are similar to lanes 1–3 with the exception that the Erk3 source was first dephosphorylated by λ phosphatase (lambda PPase) followed by inactivation of the phosphatase by the addition of sodium orthovanadate. WB, Western blot.
