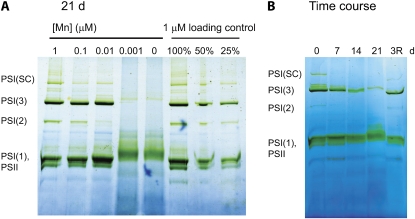
Figure 5.
BN-PAGE analysis of the oligomeric state of membrane protein complexes. A, Reorganization of thylakoid membrane complexes in response to changes in Mn bioavailability. Cultures were grown on medium containing 0 to 1 μm Mn for 21 d. Membrane protein complexes were solubilized using a ratio of 0.03 g of n-dodecylmaltoside to 1 g of chlorophyll. Each sample contained 5 μg of chlorophyll. The identification of proteins in the different membrane complexes [PSII, PSI(1), PSI(2), PSI(3), and PSI(SC) supercomplexes] was performed by peptide mass spectrometry. Full details of the tryptic peptides identified in each band can be found in Supplemental Table S2. As a loading control, 100%, 50%, and 25% of the 1 μm samples were run alongside of the 0 μm sample. Based on these controls, we could estimate that the PSI trimer content in the 0 μm sample is considerably smaller than 25% of the 1 μm trimer content. B, Time course of the reorganization of thylakoid membrane complexes during the transition in and out of Mn limitation. Mn-sufficient cells were transferred to YBG11-0. Samples were taken and analyzed by BN-PAGE over a 0- to 21-d period, as indicated. Immediately after the last sample was collected on day 21, MnCl2 was added to the remaining cells to a final concentration of 1 μm. The final sample was harvested 3 d after Mn repletion (3R). [See online article for color version of this figure.]