Table 5.
Synthesis of Exocyclic Alkenes (Suzuki and coworkers, 1992)a
| Entry | Haloalkene | Product | Yield (%) |
|---|---|---|---|
| 1 | 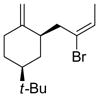 |
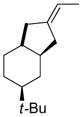 |
68 |
| 2 | 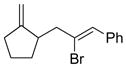 |
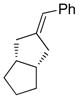 |
83 |
| 3 | 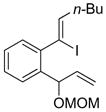 |
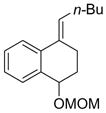 |
51 |
| 4 | 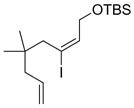 |
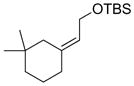 |
60 |
Hydroboration was carried out with 9-BBN-H in THF at 0 ºC-rt. for 4 h then cross-coupled intramolecularly in the presence of Pd(dppf)Cl2 (1.5 mol%) and NaOH (3.0 equiv).
