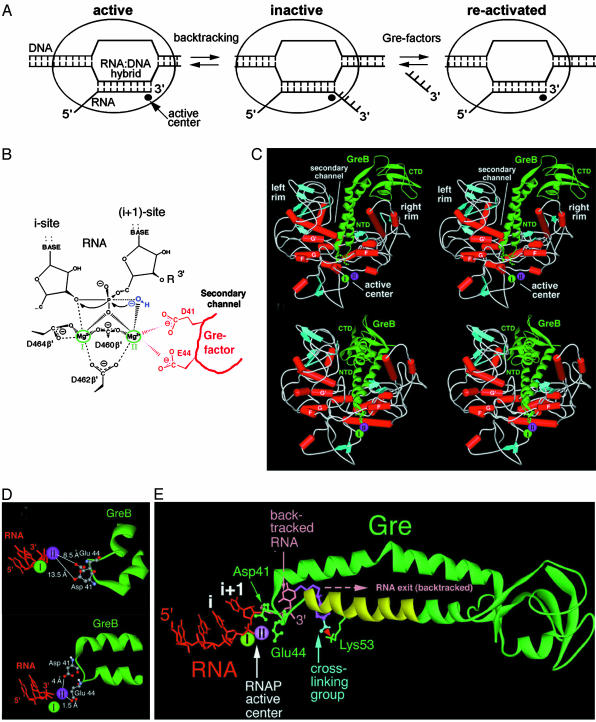
Fig. 1.
Mechanism of RNA cleavage in TEC assisted by Gre factors. (A) Schematic representation of TEC undergoing arrest and rescue. (B) The two-metal model of the active center (13) for the reaction of RNA cleavage assisted by Gre protein. RNA residues flanking the scissile phosphate occupy i and i + 1 sites of the active center. R symbolizes the extruded 3′ single-stranded RNA segment in backtracked TEC. Two Mg2+ ions required for catalysis are circled in green, coordinated by three Asp residues of RNAP β′-subunit. The attacking water molecule is shown in blue. The two acidic residues of Gre factors, which according to the present study recruit Mg-II, are represented in red. The relative orientation and distance between the interacting groups and residues is in accordance with x-ray structure and molecular modeling. (C) Docking of GreB factor into RNAP secondary channel (stereoview for crossed eyes) based on the low-resolution structure (14) of the Gre–RNAP complex (Upper) and the results of the present study (Lower). Green, GreB; white (segments with no particular secondary structure), RNAP (only relevant sections of β′-subunit are shown); red, α-helices; cyan, β-strands; light green, Mg-I; magenta, Mg-II. The residues Asp-41 and Glu-44 of Gre factor are shown in ball-and-stick representation. The helices are letter-marked reflecting the evolutionary conserved motifs of β′-subunit. The right and left rims represent the opposite sides of the rim of the secondary channel. (Upper) The N-terminal domain of Gre (NTD) is tilted ≈45° relative the bottom of the secondary channel, whereas the C-terminal domain (CTD) contacts the right part of the rim (β′ residues 960-1010; T. aquaticus numbering). (Lower) The NTD is nearly parallel to the bottom of the secondary channel, and CTD is submerged into the secondary channel. (D) Relative position of RNA, catalytic Mg2+ ions, and Asp-41, Glu-44 residues of Gre B in the active center of RNAP according to the present model (Lower) and the low-resolution cocrystal (Upper) (14). (E) Structural model of crosslinking of Gre factor to RNA in backtracked TEC. Green, GreB; red, RNA in RNA/DNA hybrid; pink, the disengaged 3′ terminal nucleotide; magenta, the crosslinking spacer; cyan, the crosslinking group; light green, Mg-I; light magenta, Mg-II. The catalytic residues of Gre factor D41 and E44 and the crosslinked Lys-53 are indicated. The red arrow indicates crosslink. The region of Gre where the crosslink in TEC from 3′ RNA terminus was mapped previously (19) is highlighted in yellow.