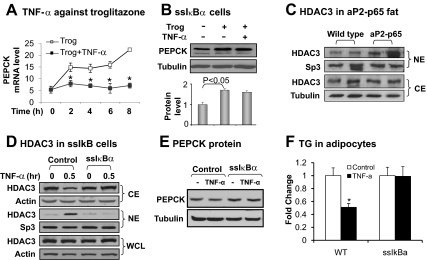
Fig. 3.
Inhibition of TNF-α activity in ssIκBα 3T3-L1 cells. A, Inhibition of troglitazone activity by TNF-α. The cells were treated with troglitazone (Trog; 10 μm) with or without TNF-α (20 ng/ml) for different times as indicated. Pepck mRNA was determined by qRT-PCR. B, Inhibition of TNF activity in ssIκBα-3T3-L1. The cells were treated with troglitazone (10 μm) with or without TNF-α (20 ng/ml) for 6 h. PEPCK protein was determined in the whole-cell lysate by Western blot. The PEPCK protein signal was determined by densitometry and normalized with tubulin protein level. C, HDAC3 in cytoplasm (CE) and nuclear (NE) fractions of epididymal white adipose tissue of control wild-type and aP2-p65 mice. HDAC3 protein was determined in the CE or NE by Western blot. Transcription factor Sp3 was a loading control for NE and tubulin was the loading control for CE. D, HDAC3 nucleus translocation. The CE and NE were made from ssIκBα or control adipocytes after TNF-α treatment for 30 min. HDAC3 protein was determined in the CE, NE, and whole-cell lysate (WCL) in an immunoblot. Sp3 was a loading control for NE and actin was the loading control for CE and the whole-cell lysate. E, PEPCK protein. In differentiated 3T3-L1 adipocytes, the PEPCK protein was examined after TNF-α treatment for 4 h. F, TG in differentiated 3T3-L1. Triglyceride was extracted and quantified in differentiated 3T3-L1 adipocytes, which were treated with TNF-α during differentiation. TG was expressed in fold change with activity in the vehicle control for 1. In this figure each bar represents mean ± sem (n = 3). *, P < 0.05.