Abstract
In the central nervous system, adenosine and ATP play an important role in regulating neuronal activity as well as controlling other neurotransmitter systems such as GABA, glutamate, and dopamine. Ethanol increases extracellular adenosine levels that regulate the ataxic and hypnotic/sedative effects of ethanol. Interestingly, ethanol is known to increase adenosine levels by inhibiting an ethanol-sensitive adenosine transporter, ENT1 (equilibrative nucleoside transporter type 1). Ethanol is also known to inhibit ATP-specific P2X receptors, which might result in such similar effects as those caused by an increase in adenosine. Adenosine and ATP exert their functions through P1 (metabotropic) and P2 (P2X-ionotropic and P2Y-metabotropic) receptors, respectively. Purinergic signaling in cortex-striatum-VTA has been implicated in regulating cortical glutamate signaling as well as VTA dopaminergic signaling, which regulates the motivational effect of ethanol. Moreover, several nucleoside transporters and receptors have been identified in astrocytes, which regulate not only adenosine-ATP neurotransmission, but also homeostasis of major inhibitory-excitatory neurotransmission (i.e. GABA or glutamate) through neuron-glial interactions. This review will present novel findings on the implications of adenosine and ATP neurotransmission in alcohol use disorders.
Keywords: Adenosine, ATP, Alcoholism, Purinergic, Signaling, Neurotransmission
Introduction
In 1972, a purine nucleotide, ATP (adenosine 5'-triphosphate), began to be recognized as a neurotransmitter. As shown in Fig. 1, adenosine is synthesized from ATP and has been known as a neurotransmitter or neuromodulator since it alters neuronal activity (Burnstock, 1972; Burnstock, 2008). In the late 1970's, adenosine receptors have been identified as P1 receptors, which are selectively antagonized by low concentrations of methylxanthines such as caffeine and theophylline (Burnstock, 2008). Currently, four well-characterized G-protein coupled adenosine receptors, A1, A2A, A2B, and A3 in the brain are known to mediate the physiological function of adenosine (Fredholm et al., 2005). Adenosine signaling has been implicated in the pathophysiology of many central nervous system (CNS) disorders including sleep disorders, anxiety, and alcoholism (Burnstock, 2008; Dunwiddie and Masino, 2001; Fredholm, 2010; Fredholm et al., 2005). Furthermore, interaction between adenosine receptors and other G-protein coupled receptors in the striatum regulates motor and motivational behaviors (Ferre et al., 2008; Ferre et al., 1997; Ferre et al., 2002; Ferre et al., 1996).
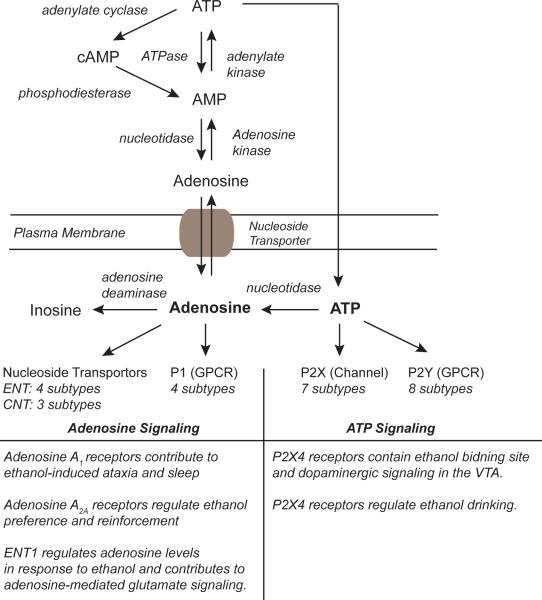
Fig. 1.
Metabolic pathways and receptors of purinergic signaling involved in alcohol use disorders. In the cytosol, adenosine is synthesized from AMP by nucleotidase activity and transported to extracellular region via nucleoside transporter. Among several nucleoside transporters, ENT1 is known to regulate adenosine levels in response to ethanol. Adenosine is also converted from ATP extracellularly by ecto-nucleotidase activity. Extracellular adenosine binds to 4 different G-protein coupled adenosine receptors and is known to mediate ethanol-induced ataxia and sleep. ATP interacts with both ion channel named P2X receptors and G-protein coupled P2Y receptors. Among these, P2X4 receptors contain an ethanol-binding site (see Figure 2) and regulate ethanol drinking. cAMP, cyclic adenosine monophosphate; ENT, equilibrative nucleoside transporter; CNT, concentrative nucleoside transporter; VTA, ventral tegmental area.
Ataxia is the earliest and most conspicuous physical manifestation of acute ethanol consumption. The striking similarity between the pharmacology of adenosine and ethanol provided strong circumstantial evidence for an adenosinergic modulation of CNS effects of ethanol. Dar and his colleagues first reported a functional relationship between adenosine and ethanol because dipyridamole, an ENT1 inhibitor, promoted ethanol-induced ataxia and sleep (Dar et al., 1983). Extracellular or synaptic adenosine levels are mainly regulated by nucleoside transporters (Baldwin et al., 1999; Sawynok and Liu, 2003). Among several nucleoside transporters, ENT1 (equilibrative nucleoside transporter type 1) regulates extracellular adenosine levels in response to ethanol treatment in cultured cells (Chen et al., 2010; Choi et al., 2004; Nagy et al., 1990).
ATP-gated P2X receptors (P2XRs) are a superfamily of ligand-gated ion-channels (Bantel et al., 2002; Khakh et al., 2001) that are becoming a focus of investigation in alcohol studies. Currently, seven subunits of the P2XRs have been identified (P2X1–P2X7) that form homo- or heteromeric channels (e.g., P2X1/4, P2X1/5, P2X2/3, P2X4/6) when expressed in Xenopus oocytes or mammalian cell lines (Lalo et al., 2008; Le et al., 1998; Lewis et al., 1995; Nicke et al., 2005). Each P2XR subunit consists of two alpha-helical transmembrane (TM) segments, a large extracellular domain (ectodomain), and an intracellular location of amino and carboxy terminals (North, 2002). Recent crystallographic investigations on zebra fish P2X4Rs (Kawate et al., 2009) confirmed previous predictions that functional P2XRs result from the assembly of three subunits (Aschrafi et al., 2004; Jiang et al., 2003).
BRAIN ADENOSINERGIC A1 MODULATION OF ETHANOL-INDUCED ATAXIA
Using long-sleep (LS) and short-sleep (SS) mice as genetic models with differential sensitivity to the soporific effects of ethanol, Proctor and Dunwiddie (1984) reported that LS mice were sensitive not only to ethanol's soporific effects, but also to the sedative effects of an adenosine agonist, further supporting that adenosine signaling is involved in ethanol intoxication. Other investigators confirmed and elegantly extended these studies that demonstrated a role of adenosine in alcoholism (Gordon et al., 1986; Nagy et al., 1990). A key mechanism in the behavioral effects of ethanol involves inhibition of alcohol-sensitive ENT1 (Nagy et al., 1990). This increases the extraneuronal adenosine levels that activate adenosine receptors and is known to mediate acute and chronic effects of ethanol (Diamond and Gordon, 1994; Dunwiddie and Masino, 2001). Interestingly, mice lacking ENT1 stayed on the rotarod significantly longer compared to wild-type mice when mice were given 1.0 or 1.5 g/kg ethanol (i.p.) (Choi et al., 2004). Since ENT1 null mice showed reduced A1 receptor function (Choi et al., 2004), diminished A1 receptor may mediate resistance to ethanol-induced ataxia.
Dar and his colleagues have confirmed the A1 adenosinergic modulation of ethanol-induced ataxia in male CD-1 mice and Sprague-Dawley rats, using intraperitoneal (ip), intracerebroventricular (icv), intracerebellar (ICB), intrastriatal (IST), and intramotorcortical (IMC) administration of adenosine agonists/antagonists. The sensitivity of the cerebellum to ethanol has been associated with cAMP-response-element-binding protein (CREB) transcription activity and cerebellar activation that plays a role in alcoholism (Acquaah-Mensah et al., 2006). It is the significance of cerebellum in alcoholism that directed our focus to the study of ethanol-induced ataxia, although striatum (Meng et al., 1997) and motor cortex (Barwick and Dar, 1998) also mediate ethanol-induced ataxia.
Direct ICB infusion of adenosine A1-, A1/A2A- and A2A-selective agonists/antagonists, including N6–cyclohexyl-adenosine (CHA; an adenosine A1 agonist) and 5'-N-ethylcarboxamido-adenosine (NECA; an agonist with A1/A2A affinity), markedly accentuated ethanol-induced ataxia that was blocked by adenosine A1 antagonist, 8-cyclopentyl-1, 3-dipropylxanthine (DPCPX) (Dar, 1990; Dar, 1998). The role of A1 receptors in the modulation of ethanol-induced ataxia was further supported indirectly by the results of experiments involving microinfusion of 2-p-(2-carboxyethyl) phenethylamino-5'-N-carboxaminoadenosine (CGS-21680; an adenosine A2A-selective agonist) into the rat motor cortex (Barwick and Dar, 1998) and mouse striatum (Dar, 2001). In spite of its high A2A-selectivity [Ki= 2600 nM (A1); Ki = 15nM (A2A)], at least a 25-fold higher dose of CGS-21680 was required compared to CHA, to produce comparable accentuation of ethanol-induced ataxia (Dar, 2001). The accentuation by CGS-21680 was totally abolished by pretreatment with DPCPX indicating A1- and not A2A-receptor modulation (Dar, 2001). Further evidence for the modulation of ethanol-induced ataxia by A1- and not A2A-receptor was provided when intramotor cortex microinfusion of a high dose (4 nmol) of CGS-21680 accentuated ethanol-induced ataxia that was abolished by DPCPX but not by A2A-selective antagonist, 8-(3-chlorostyryl) caffeine (Barwick and Dar, 1998). Thus, the accentuation by CGS-21680 at such a high dose presents the possibility that it is modulating ethanol-induced ataxia through a non-selective effect at the adenosine A1 receptor. Finally, pretreatment with adenosine A1 antisense RNA blocked the CHA-induced accentuation of ethanol-induced ataxia, indicating that an inhibition of A1 receptor expression is associated with a decreased ethanol response (Dar and Mustafa, 2002).
The ethanol-induced cerebellar ataxia was functionally related to: (i) an increase in the maximum number of adenosine A1 receptors (Clark et al., 1993); (ii) an inhibition of adenosine uptake (Clark and Dar, 1989b) via inhibition of alcohol-sensitive ENT1; (iii) an increase in adenosine release (Clark and Dar, 1989a); and, (iv) a decrease in glutamate release in an adenosine-sensitive manner (Clark and Dar, 1989c). The A1 adenosine receptors are highly expressed in the cerebellar granule cells, their axons, and axonal terminals (Dar, 1997). Since A1 receptors are coupled with the Gi/Go protein, the adenylate cyclase/cAMP/PKA signaling system has been implicated in the behavioral responses of ethanol (Newton and Messing, 2006). This signaling pathway participates in ethanol-induced ataxia because ethanol-induced ataxia is augmented by pretreatment with miconazole, an inhibitor of adenylate cyclase (Dar, 1997), and attenuated by forskolin, a stimulator of adenylate cyclase or by cAMP/cpt-cAMP (Dar, 1997). In addition, ethanol-induced ataxia is positively correlated with increased chloride uptake in the striatal microsacs, which is also regulated by adenosine A1 receptors (Meng et al., 1997). Since GABAA agonist (muscimol) activated ethanol-induced ataxia while GABAA antagonist (bicuculline) has an opposite effect (Dar, 2006), GABA receptor-mediated chloride influx into the cells contributes to ethanol-induced ataxia. Interestingly, ethanol is also known to potentiate GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability (Carta et al., 2004). The GABA released at the granule cell synapse dampens the firing of granule cells, which are the only excitatory cells within the cerebellar cortex (Voogd and Glickstein, 1998). Consequently, in the deficit of this excitatory signal, the GABAergic Purkinje cells inhibit the deep cerebellar nuclei, thereby resulting in ethanol-induced ataxia.
The inhibition of ENT1 by acute ethanol increases extracellular adenosine levels (Nagy et al., 1990; Nagy et al., 1989), which activates A1 receptors and decreases glutamate release at parallel fibers to the Purkinje cell synapse. This process leads to the expression of ethanol-induced cerebellar ataxia. Ethanol also inhibits nitric oxide synthase (NOS), causing decreased nitric oxide (NO) levels (Al-Rejaie and Dar, 2006a; Al-Rejaie and Dar, 2006b). Cerebellar NOS is located in the granule and basket cells from which soluble NO diffuses to Purkinje cells (Fedele et al., 1998). The Purkinje cells exhibit strong immunoreactivity for guanylyl cyclase (Marcoli et al., 2006). Thus, NO would stimulate cGMP production in the Purkinje cells, resulting in decreased Purkinje cell firing. The cerebellar glutamate system appears to regulate NO-cGMP signaling as elucidated in our previous studies (Al-Rejaie and Dar, 2006a; Al-Rejaie and Dar, 2006b). As Purkinje cells represent the only inhibitory output in the cerebellar cortex, any event leading to the depression of Purkinje cell firing would cause a decrease in GABAergic transmission within the Purkinje cells and the consequent attenuation of ethanol-induced ataxia. Thus, the overall effect of ethanol is to suppress excitatory glutamatergic transmission and increase GABAergic inhibitory firing by enhancing inhibitory function of Purkinje cells on the deep cerebellar nuclei, all of which lead to ataxia.
A ROLE OF ADENOSINE AND THE WAKE-PROMOTING BASAL FOREBRAIN IN MEDIATING THE SOMNOGENIC EFFECTS OF ETHANOL
It is known that ethanol intake has significant effects on sleep (Brower, 2001; Roehrs and Roth, 2001). However, the cellular substrates responsible for mediating the effects of ethanol on sleep are unknown. Strong and consistent evidence suggest that adenosine, a somnogen, is a key mediator of many behavioral and neuronal responses to ethanol including ataxia, anxiety, tremors, and seizures (Barwick and Dar, 1998; Batista et al., 2005; Concas et al., 1996; Dar, 2006; Dunwiddie and Masino, 2001; Hack and Christie, 2003; Jarvis and Becker, 1998; Kaplan et al., 1999; Newton and Messing, 2006; Phan et al., 1997; Prediger et al., 2006). Acute exposure to ethanol inhibits adenosine reuptake via ENT1 in cell cultures. Chronic ethanol exposure down-regulates ENT1 expression (Krauss et al., 1993; Nagy et al., 1990). ENT1 null mice display decreased adenosinergic tone, resulting in increased ethanol consumption in addition to reduced hypnotic (loss of righting reflex) and ataxic responses to ethanol. In contrast, treatment with an A1 receptor agonist decreased ethanol consumption in ENT1 null mice (Choi et al., 2004).
Previous studies suggest that adenosine is a homeostatic regulator of sleep (Portas et al., 1997; Radulovacki et al., 1984; Thakkar and Mallick, 1996; Thakkar et al., 2003b). During prolonged wakefulness, adenosine, a byproduct of metabolism, accumulates in the wake-promoting basal forebrain (BF) region that includes the horizontal diagonal band, the substantia innominata and the magnocellularis pre-optic nuclei (HDB/SI/MCPO) (Murillo-Rodriguez et al., 2004; Porkka-Heiskanen et al., 1997). Increased adenosine in the BF acts via A1 receptors to inhibit the BF cholinergic and non-cholinergic wake-promoting (GABAergic and glutamatergic) neurons (Alam et al., 1999; Arrigoni et al., 2006; Thakkar et al., 2003a; Thakkar et al., 2003b). Inhibition of BF wake-promoting neurons results in the transition from wakefulness to sleep (Basheer et al., 2004; McCarley, 2007). Lesions of the BF cholinergic neurons abolish prolonged wakefulness, induce an increase in adenosine release, and attenuate the homeostatic response to sleep deprivation (Blanco-Centurion et al., 2006; Kalinchuk et al., 2008; Kaur et al., 2008).
Recent studies suggest that acute intragastric administration of ethanol (3 g/kg) in freely behaving Sprague-Dawley rats produces a significant increase in slow wave sleep (non-rapid eye movement sleep) with a concomitant decrease in wakefulness during the first 12 hours after ethanol treatment. Rapid eye movement (REM) sleep remains unaffected. Bilateral microinjections localized in the HDB/SI/MCPO [see Figure 4 in (Thakkar et al., 2010)] of a selective A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), significantly attenuates the sleep-inducing effects of ethanol, suggesting a role of A1 receptor in ethanol-induced sleep. Furthermore, intragastric administration of ethanol (3 g/kg) causes a significant decrease in the number of BF wake-promoting neurons with c-Fos immunoreactivity, suggesting ethanol-induced inhibition of BF wake-promoting neurons (Thakkar et al., 2010). While these results suggest that adenosinergic mechanisms may play an essential role in ethanol-induced sleep promotion by inhibiting the wake-promoting neurons of the BF, the confirmatory test demonstrated that local microdialysis perfusion of ethanol induces a significant and dose-dependent increase in extracellular levels of adenosine in the BF (Thakkar et al., 2010). As expected, local administration of ethanol in the BF region is known to increase the duration of sleep (Mendelson, 2001). These recent results suggest that ethanol causes an increase of extracellular adenosine in the BF. Increased extracellular adenosine inhibits the BF wake-promoting neurons via A1 receptor to promote sleep, which is a key mechanism responsible for sleep induction following acute ethanol intake. Since adenosine is a homeostatic regulator of sleep, and since ethanol has no effect on the circadian timing of sleep onset at lights-on [see Figure 2 in Thakkar et. al., 2010], acute ethanol intake may affect the homeostatic, but not the circadian regulation, of sleep.
INTERACTION OF ADENOSINE RECEPTORS WITH OTHER G-PROTEIN COUPLED RECEPTORS AND ETHANOL PREFERENCE
It is evident that adenosine A2A and dopamine D2 receptors interact with each other on the plasma membranes or in the cytoplasm through signaling molecules (Ferre et al., 1991; Short et al., 2006a). Similarly, adenosine A1 and dopamine D1 receptor can also cross talk (Ferre et al., 1996). Especially, dimerization of A2A receptors with D2 receptors in the striatum allows the A2A to influence reward-related behavior through modulation of adenylate cyclase activity (Ferre et al., 2008; Ferre et al., 1994). Contrary to the antagonistic actions of these receptors under normal circumstances, in the presence of ethanol, the two receptors can have synergistic activity through Gβγ which increases cAMP levels and PKA activity (Mailliard and Diamond, 2004; Yao et al., 2002; Yao et al., 2003). In rats, A2 antagonist 3,7-dimethyl-1-propargylxanth (DMPX) attenuated ethanol self-administration (Arolfo et al., 2004; Thorsell et al., 2007), indicating that modulation of A2A receptor plays an essential role in ethanol preference. On the other hand, A2A agonists have been shown to reduce ethanol-withdrawal responses (Kaplan et al., 1999). Paradoxically, mice lacking the A2A receptor demonstrate reduced sensitivity to the hypnotic effects of alcohol, increased alcohol consumption, and reduced alcohol-withdrawal seizures (El Yacoubi et al., 2001; Naassila et al., 2002).
Adenosine A2A receptor is involved in the synergistic interaction between mu-opioid and cannabinoid CB1 receptors in heroin-seeking behaviors (Yao et al., 2006). Since mu-opioid receptors regulate alcohol preference (Herz, 1997) and a genetic variant of human mu-opioid receptor (OPRM1) is a predictor of naltrexone efficacy, an FDA approved medication for alcoholism (Anton et al., 2008), dysregulation of A2A receptor might be an genetic factor of alcohol use disorders in humans. In addition, adenosine A2A receptors also synergistically interact with mGluR5 glutamate receptors in the striatum (Ferre et al., 2002). Since mGluR5 glutamate receptors have an essential role in alcohol preference, especially in the nucleus accumbens (Besheer et al., 2009; Hodge et al., 2006; Olive et al., 2005), indirect regulation of mGluR5 by A2A receptors may also contribute to alcohol use disorders. Co-activation of adenosine A2A and mGluR5 receptors in the neostriatal slices from mice is known to synergistically increase phosphorylation of dopamine- and cAMP-regulated phosphoprotein (DARPP-32) on threonine 34 (Nishi et al., 2003), which promotes inhibition of protein phosphatase-1 (Greengard, 2001; Greengard et al., 1999). Interestingly, mice lacking DARPP-32 self-administer less ethanol than wild-type mice (Risinger et al., 2001), suggesting that co-inhibiting adenosine A2A and mGluR5 receptors might inhibit DARPP-32 and reduce alcohol consumption. The effect of mGluR5 receptor agonist (CHPG) on GABA release in the ipsilateral ventral pallidum was strongly potentiated by co-perfusion with the adenosine A2A agonist, CGS21680 (Diaz-Cabiale et al., 2002), also demonstrating that synergistic interaction of these receptors promote striatal neuronal activity. On the other hand, motor stimulating effect of mGluR5 receptor antagonist (MPEP) is stimulated by adenosine A2A receptor antagonist (KW-6002), which indicates an in vivo functional interdependence of these receptors (Kachroo et al., 2005). Consistently, the co-administration of sub-threshold doses of an adenosine A2A receptor antagonist (SCH 58261) with an mGlu5 receptor antagonist (MTEP) reduced both alcohol self-administration and cue-induced reinstatement of alcohol-seeking in rats (Adams et al., 2008), suggesting that a combinational drug strategy for treating alcoholism could be considered.
A ROLE OF ENT1 IN REGULATING GLUTAMATE NEUROTRANSMISSION IN REWARD CIRCUITRY
Extracellular or synaptic adenosine levels are mainly regulated by nucleoside transporters (Baldwin et al., 1999; Sawynok and Liu, 2003). Two main plasma membrane transporter families have been characterized. Equilibrative nucleoside transporters (ENTs) mediate nucleoside transport bi-directionally depending on the concentration gradient across the plasma membrane, whereas concentrative nucleoside transporters (CNTs) mediate inwardly directed transport driven by the sodium electrochemical gradient (Baldwin et al., 1999). Three ENT subtypes have been cloned and characterized (Hyde et al., 2001). ENT1 is sensitive to nanomolar concentrations of nitrobenzylthioinosine (NBTI), whereas ENT2 is resistant to NBTI up to 1 mM (Crawford et al., 1998; Griffiths et al., 1997a; Griffiths et al., 1997b; Yao et al., 1997). ENT1 and ENT2 are widely expressed in the CNS (Anderson et al., 1999a; Anderson et al., 1999b; Jennings et al., 2001). ENT3 appears to be expressed outside of the nervous system, and its pharmacological properties have not yet been fully characterized (Hyde et al., 2001). ENT1 and ENT2 are about 50% homologous in amino acid sequence and contain 11 putative transmembrane domains.
Nucleoside transport across the plasma membrane is one of several factors that regulate extracellular adenosine concentrations, which in the brain range from 25–250 nM under basal conditions (Dunwiddie and Masino, 2001). These are sufficient to tonically activate a significant fraction of high affinity A1 and A2A receptors. Among several nucleoside transporters, ENT1 regulates extracellular adenosine levels in response to acute ethanol treatment in cultured cells (Nagy et al., 1990). Acute ethanol treatment increases extracellular adenosine in cultured cells by selectively inhibiting ENT1 while chronic ethanol exposure decreases ENT1 expression and no longer increases levels of extracellular adenosine, which can be viewed as a cellular model of tolerance (Nagy et al., 1990). Since mice lacking ENT1 exhibit reduced ataxic/hypnotic effects to acute ethanol exposure (Choi et al., 2004) and lowered initial sensitivity (Chen et al., 2010), ENT1 null mice appear to mimic a chronic ethanol treated status. Consistently, ENT1 null mice consume more alcohol compared to wild-type littermates (Choi et al., 2004). Interestingly, mice over-expressing human ENT1 are more sensitive to the acute intoxicating effect of ethanol (Parkinson et al., 2009). Consistently, ENT1 expression appears higher in the striatum of CD1 mice compared with both C57BL/6J and C57BL/6J × CD1 mice (Short et al., 2006b). Since C57BL/6J mice display increased ethanol drinking compared to CD mice (Short et al., 2006b), ENT1 expression might be inversely correlated with ethanol consumption.
One of the neural mechanisms underlying increased ethanol preference in ENT1 null mice is attributed to increased glutamate neurotransmission in the nucleus accumbens (NAc) (Choi et al., 2004). Interestingly, inhibition of presynaptic adenosine A1 receptor is known to promote glutamate-mediated synaptic neurotransmission in the hippocampus (Manzoni et al., 1994). Consistently, in the NAc, inhibition of adenosine A1 receptor increases glutamate-evoked synaptic activity (Harvey and Lacey, 1997). Previously, we found that activation of adenosine A1 receptor reduces ethanol intake in ENT1 null mice (Choi et al., 2004), suggesting that diminished adenosine activity might be related to increased glutamate levels and increased ethanol intake. A recent study indicates that increased resistance to acute ethanol intoxication is possibly related to increased glutamate signaling in ENT1 null mice (Chen et al., 2010). Moreover, we also found that inhibition of ENT1 expression or activity reduces excitatory amino acid transporter 2 (EAAT2) expression and glutamate activity in cultured astrocytes, which might contribute to increased extracellular glutamate levels in ENT1 null mice (Wu et al., 2010).
Interestingly, hetero-dimerization of adenosine A1 and A2A receptors in striatal glutamatergic nerve terminals is known to finely tune glutamate release depending on adenosine concentrations (Ciruela et al., 2006; Quarta et al., 2004b). At lower adenosine concentration, high-affinity A1 receptor inhibits glutamate release, whereas a higher adenosine concentration stimulates glutamate release through A2A receptor (Ciruela et al., 2006). Thus, either decreased A1 receptor experession or increased A2A receptor function might lead to increased glutamate release. In addition, this interaction regulates dopamine release in the NAc as well (Quarta et al., 2004a).
Choi and his colleagues have demonstrated that altered adenosine-glutamate homeostasis in ENT1 null mice is implicated in the resistance to ethanol-induced locomotion and ataxia by NMDA antagonist, CGP37849 (Nam et al., 2010). ENT1 null mice appear less intoxicated following sequential treatment of CGP37849 and ethanol compared to wild-type littermates in a rotarod experiment. These results indicate that glutamate neurotransmission is critical in regulating the response and susceptibility of alcohol related behavior. Interestingly, a microdialysis experiment revealed that the NAc of ENT1 null mice is less sensitive to the glutamate-reducing effect of the NMDA receptor antagonist (Nam et al., 2010). These findings suggest that glutamate neurotransmission in the NAc is essential to regulate ethanol intoxication.
Increased glutamate signaling is implicated in CREB-mediated gene expression, which regulates several addictive behaviors (Kalivas, 2009; Nestler, 2001). Importantly, excessive glutamate neurotransmission is known to be associated with increased ethanol drinking (Spanagel and Kiefer, 2008; Spanagel et al., 2005). However, it seems paradoxical that both increased glutamate signaling and decreased CREB activity (Pandey et al., 2004) are associated with alcohol use disorders since increased glutamate receptor signaling is likely to increase CREB activity via several signaling mechanisms (Lonze and Ginty, 2002).
A ROLE FOR ATP-GATED P2X4 RECEPTORS IN ALCOHOL CONSUMPTION
P2XRs are widely distributed in the CNS on neurons (Surprenant and North, 2009) and on glial cells (Inoue, 2008; Trang et al., 2006). Tests using brain slice preparations, dissociated neuronal cultures, and P2XR knockout (KO) mouse models proposed possible roles for P2XRs (Surprenant and North, 2009). This includes learning and memory (Labrousse et al., 2009; Sim et al., 2006; Wang et al., 2004), depression and anxiety (Basso et al., 2009), pain perception (Honore et al., 2006; Jarvis et al., 2002; Tsuda et al., 2009; Ulmann et al., 2008), and vascular tone (Yamamoto et al., 2006). In addition, P2XRs have been suggested to play a role in hormonal control of temperature regulation, food and water intake, sexual behavior, and emotional responses (Stojilkovic, 2009), which are also implicated in alcohol use disorders.
The mesolimbic dopamine (DA) system plays an important role in ethanol consumption, ethanol addiction, and reinforcement (Gonzales et al., 2004; Spanagel and Weiss, 1999). P2XRs have been identified on both neurons and glia in the mesolimbic DA system (Heine et al., 2007). A recent study indicates a functional role of the P2XRs in the ventral tegmental area (VTA). P2XRs modulate ethanol's effect on GABAergic synaptic transmission in the ventral tegmental area (VTA) (Xiao et al., 2008). Taken together, these findings suggest that P2XRs may have an important role in modulating the activity of dopaminergic neurons that are important for controlling ethanol intake.
P2X4Rs are the most abundant P2XR subtype expressed in the CNS (Buell et al., 1996; Soto et al., 1996), and building evidence implicates P2X4Rs in alcohol consumption. First, a recent study using a genomic/phenomic approach of ethanol consumption identified p2rx4 as one of the “candidate genes” that predisposes to varying levels of ethanol intake across the 28 recombinant inbred rat strains (Tabakoff et al., 2009). The authors concluded that interactions of the products of the candidate genes identified in the study were involved in neurobiological pathways affecting GABAergic neuronal activity in the mesolimbic DA system. Second, Davies and colleagues recently found that mice lacking the p2rx4 gene displayed significantly different alcohol consumption compared to wild type C57BL/6J or heterozygous littermates (manuscript in preparation). Collectively, these findings support the contention that P2XRs play a role in ethanol drinking.
Developing effective treatments for alcohol related disorders faces a number of challenges due to limited knowledge of the sites and mechanisms of ethanol action in the CNS. Over the past several years, Davies and his colleagues have placed a significant amount of effort focusing on sites of ethanol action in P2XRs. Studies using recombinant expression systems have found that P2X2, P2X3, and P2X4Rs expressed in Xenopus oocytes are sensitive to ethanol at intoxicating and anesthetic concentrations (Davies et al., 2005; Davies et al., 2002; Xiong et al., 2000). Also, these studies demonstrated that residues contained within the ectodomain-TM interfaces are important for causing or modulating the effects of ethanol in P2X3 and P2X4Rs (Asatryan et al., 2008; Popova et al., 2010). More specifically, in P2X4Rs, mutational analyses identified amino acid residues within the TM segments at the ectodomain-TM interface (W46, D331, M336) that are critical for ethanol action (Popova et al., 2010).
Recent work has demonstrated that the ectodomain-TM interface of P2X4Rs is also an important site for modulation of the receptor activity by ivermectin (IVM) (Jelinkova et al., 2008; Jelinkova et al., 2006; Silberberg et al., 2007). IVM is a semi-synthetic macrocyclic lactone widely employed in both animals and humans as a broad spectrum anthelmintic (Geary, 2005; Omura, 2008; Richard-Lenoble et al., 2003). The current therapeutic potential of IVM is attributed to action on a non-mammalian, glutamate-gated inhibitory chloride channel (Cully et al., 1994; Dent et al., 1997; Le et al., 1998). However, IVM can also potentiate GABAA and glycine receptors in vitro (Dawson et al., 2000; Shan et al., 2001). More recent studies in humans suggest that IVM also affects other LGICs including nicotinic acetylcholine receptor (Krause et al., 1998; Sattelle et al., 2009) and P2X4Rs (Khakh et al., 1999). Regarding P2X4Rs, IVM is used to selectively identify the participation of P2X4Rs from other P2X family members in ATP-mediated processes (Khakh et al., 1999).
Interestingly, some of the sites or regions that IVM is purported to act on have recently been reported as being important for ethanol modulation (Asatryan et al., 2008; Popova et al., 2010). Based in part of this finding, Asatryan and colleagues tested the hypothesis that IVM would reduce the sensitivity of P2X4Rs to ethanol (Asatryan et al., 2010). In agreement with this hypothesis, IVM antagonized ethanol in a concentration-dependent manner (Asatryan et al., 2010). As illustrated in Fig. 2, these findings were used to construct the first molecular model illustrating overlapping sites of action for ethanol and IVM (i.e. M336) in P2X4Rs (Asatryan et al., 2010). Taken together, the findings suggest that the ectodomain-TM interface of P2X4Rs is a site of action and/or modulation for both ethanol and IVM. In addition, the newly identified alcohol pocket may represent a potential target for the development of medication for prevention and treatment of alcohol use disorders.
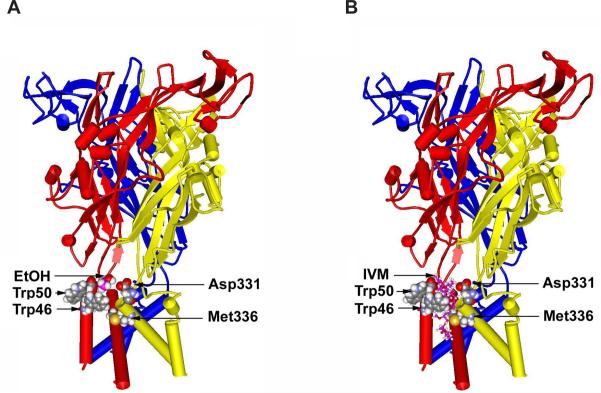
Fig. 2.
Molecular model of the rat P2X4R reveals a putative ethanol and IVM pocket. The model was built by threading the edited primary sequence onto the X-ray crystal structure of zebra fish P2X4R (Kawate et al., 2009). (A) A side view of the rat P2X4R showing the ectodomain and the six alpha helices of TM1 and TM2 segments of 3 different P2X4R subunits. Residues W46, W50 in the first alpha helix of one subunit as well as D331 and M336 in the final alpha helix of the adjacent subunit form a pocket that demonstrates a good fit for a molecule of ethanol (in pink) at the same scale. (B) A similar view of the rat P2X4R, but with a model of IVM (rendered in balls and sticks) inserted into a putative binding site in a position between the alpha helices like that described in nicotinic acetylcholine receptors (Sattelle et al., 2009). Figure taken from (Asatryan et al., 2010).
FUTURE DIRECTIONS
It is evident that adenosine and ATP signaling are implicated in several aspects of alcohol use disorders (Fig. 1). In addition to significant recent advances in molecular and neurobiological basis of purinergic metabolism and signaling, further experiments will be required to determine the causal relationship between adenosine and ATP signaling in neuron-glial interactions, and how the interaction finely regulates other major neurotransmitters such as dopamine, GABA, and glutamate signaling in circuitry levels. Considering the apparent function of adenosine signaling as a main contributor for initial ethanol-induced intoxication or tolerance, the molecular basis of ethanol dependence in the context of tolerance to ethanol intoxication still needs to be determined. Reduced ethanol intoxication is a key determinant of increased ethanol consumption (Offenhauser et al., 2006); therefore, unraveling precise adenosine signaling would be essential to identify addictive properties of ethanol. Furthermore, detailed molecular mechanisms underlying involvement of adenosine signaling in ethanol-induced sleep processes or associated disorders will be critical to understand a hangover or negative reinforcing effects of ethanol. Finally, recent studies on P2X4 and its direct interaction with ethanol open a new possibility. Additional preclinical experiments with ivermectin (IVM) in animal models will be required to lead on to clinical trials.
ACKNOWLEDGEMENTS
We thank D. Frederixon, S. Johng for editing the manuscript. This project was funded by the Samuel Johnson Foundation for Genomics of Addiction Program at Mayo Clinic to D.S.C., by the Harry S. Truman Memorial Veterans Hospital to M.M.T. and by grants from the National Institutes of Health (NIH) to D.S.C. (AA015164, AA018779, AA017830-Project 1), to M.S.D. (AA0701), to M.M.T. (NS059831 and RAA017472A), to L.A. (AA017243, Project 4), to D.L.D. (AA013922 and Integrative Neurosciences Initiative on Alcoholism AA013517, Project-4).
REFERENCES
- Acquaah-Mensah GK, Misra V, Biswal S. Ethanol sensitivity: a central role for CREB transcription regulation in the cerebellum. BMC Genomics. 2006;7:308. doi: 10.1186/1471-2164-7-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams CL, Cowen MS, Short JL, Lawrence AJ. Combined antagonism of glutamate mGlu5 and adenosine A2A receptors interact to regulate alcohol-seeking in rats. Int J Neuropsychopharmacol. 2008;11:229–241. doi: 10.1017/S1461145707007845. [DOI] [PubMed] [Google Scholar]
- Al-Rejaie S, Dar MS. Behavioral interaction between nicotine and ethanol: possible modulation by mouse cerebellar glutamate. Alcohol Clin Exp Res. 2006a;30:1223–1233. doi: 10.1111/j.1530-0277.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- Al-Rejaie S, Dar MS. Possible role of mouse cerebellar nitric oxide in the behavioral interaction between chronic intracerebellar nicotine and acute ethanol administration: observation of cross-tolerance. Neuroscience. 2006b;138:575–585. doi: 10.1016/j.neuroscience.2005.11.034. [DOI] [PubMed] [Google Scholar]
- Alam MN, Szymusiak R, Gong H, King J, McGinty D. Adenosinergic modulation of rat basal forebrain neurons during sleep and waking: neuronal recording with microdialysis. J Physiol. 1999;521:679–690. doi: 10.1111/j.1469-7793.1999.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Baldwin SA, Young JD, Cass CE, Parkinson FE. Distribution of mRNA encoding a nitrobenzylthioinosine-insensitive nucleoside transporter (ENT2) in rat brain. Brain Res Mol Brain Res. 1999a;70:293–297. doi: 10.1016/s0169-328x(99)00164-3. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Xiong W, Geiger JD, Young JD, Cass CE, Baldwin SA, Parkinson FE. Distribution of equilibrative, nitrobenzylthioinosine-sensitive nucleoside transporters (ENT1) in brain. J Neurochem. 1999b;73:867–873. doi: 10.1046/j.1471-4159.1999.0730867.x. [DOI] [PubMed] [Google Scholar]
- Anton RF, Oroszi G, O'Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arolfo MP, Yao L, Gordon AS, Diamond I, Janak PH. Ethanol operant self-administration in rats is regulated by adenosine A2 receptors. Alcohol Clin Exp Res. 2004;28:1308–1316. doi: 10.1097/01.alc.0000139821.38167.20. [DOI] [PubMed] [Google Scholar]
- Arrigoni E, Chamberlin NL, Saper CB, McCarley RW. Adenosine inhibits basal forebrain cholinergic and noncholinergic neurons in vitro. Neuroscience. 2006;140:403–413. doi: 10.1016/j.neuroscience.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Asatryan L, Popova M, Perkins D, Trudell JR, Alkana RL, Davies DL. Ivermectin Antagonizes Ethanol Inhibition in P2x4 Receptors. J Pharmacol Exp Ther. 2010;334:720–728. doi: 10.1124/jpet.110.167908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatryan L, Popova M, Woodward JJ, King BF, Alkana RL, Davies DL. Roles of ectodomain and transmembrane regions in ethanol and agonist action in purinergic P2X2 and P2X3 receptors. Neuropharmacology. 2008;55:835–843. doi: 10.1016/j.neuropharm.2008.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschrafi A, Sadtler S, Niculescu C, Rettinger J, Schmalzing G. Trimeric architecture of homomeric P2X2 and heteromeric P2X1+2 receptor subtypes. J Mol Biol. 2004;342:333–343. doi: 10.1016/j.jmb.2004.06.092. [DOI] [PubMed] [Google Scholar]
- Baldwin SA, Mackey JR, Cass CE, Young JD. Nucleoside transporters: molecular biology and implications for therapeutic development. Molecular Medicine Today. 1999;5:216–224. doi: 10.1016/S1357-4310(99)01459-8. [DOI] [PubMed] [Google Scholar]
- Bantel C, Childers SR, Eisenach JC. Role of adenosine receptors in spinal G-protein activation after peripheral nerve injury. Anesthesiology. 2002;96:1443–1449. doi: 10.1097/00000542-200206000-00025. [DOI] [PubMed] [Google Scholar]
- Barwick VS, Dar MS. Adenosinergic modulation of ethanol-induced motor incoordination in the rat motor cortex. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:587–607. doi: 10.1016/s0278-5846(98)00025-6. [DOI] [PubMed] [Google Scholar]
- Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Basso AM, Bratcher NA, Harris RR, Jarvis MF, Decker MW, Rueter LE. Behavioral profile of P2X7 receptor knockout mice in animal models of depression and anxiety: relevance for neuropsychiatric disorders. Behav Brain Res. 2009;198:83–90. doi: 10.1016/j.bbr.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Batista LC, Prediger RD, Morato GS, Takahashi RN. Blockade of adenosine and dopamine receptors inhibits the development of rapid tolerance to ethanol in mice. Psychopharmacology (Berl) 2005;181:714–721. doi: 10.1007/s00213-005-0014-7. [DOI] [PubMed] [Google Scholar]
- Besheer J, Grondin JJ, Salling MC, Spanos M, Stevenson RA, Hodge CW. Interoceptive effects of alcohol require mGlu5 receptor activity in the nucleus accumbens. J Neurosci. 2009;29:9582–9591. doi: 10.1523/JNEUROSCI.2366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Centurion C, Xu M, Murillo-Rodriguez E, Gerashchenko D, Shiromani AM, Salin-Pascual RJ, Hof PR, Shiromani PJ. Adenosine and sleep homeostasis in the Basal forebrain. J Neurosci. 2006;26:8092–8100. doi: 10.1523/JNEUROSCI.2181-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ. Alcohol's effects on sleep in alcoholics. Alcohol Res Health. 2001;25:110–125. [PMC free article] [PubMed] [Google Scholar]
- Buell G, Lewis C, Collo G, North RA, Surprenant A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. Embo J. 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7:575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24:3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Nam HW, Lee MR, Hinton DJ, Choi S, Kim T, Kawamura T, Janak PH, Choi D-S. Altered glutamatergic neurotransmission in the striatum regulates ethanol sensitivity and intake in mice lacking ENT1. Behav Brain Res. 2010;208:636–642. doi: 10.1016/j.bbr.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, McMahon T, Diamond I, Bonci A, Messing RO. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat Neurosci. 2004;7:855–861. doi: 10.1038/nn1288. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortes A, Canela EI, Lopez-Gimenez JF, Milligan G, Lluis C, Cunha RA, Ferre S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M, Dar MS. Effect of acute ethanol on release of endogenous adenosine from rat cerebellar synaptosomes. J Neurochem. 1989a;52:1859–1865. doi: 10.1111/j.1471-4159.1989.tb07268.x. [DOI] [PubMed] [Google Scholar]
- Clark M, Dar MS. Effect of acute ethanol on uptake of [3H]adenosine by rat cerebellar synaptosomes. Alcohol Clin Exp Res. 1989b;13:371–377. doi: 10.1111/j.1530-0277.1989.tb00338.x. [DOI] [PubMed] [Google Scholar]
- Clark M, Dar MS. Release of endogenous glutamate from rat cerebellar synaptosomes: interactions with adenosine and ethanol. Life Sci. 1989c;44:1625–1635. doi: 10.1016/0024-3205(89)90479-7. [DOI] [PubMed] [Google Scholar]
- Clark M, Weiss SR, Post RM. Autoradiographic analysis of serotonin receptors and transporter in kindled rat brain. Neurosci Lett. 1993;161:21–26. doi: 10.1016/0304-3940(93)90130-d. [DOI] [PubMed] [Google Scholar]
- Concas A, Mascia MP, Cuccheddu T, Floris S, Mostallino MC, Perra C, Satta S, Biggio G. Chronic ethanol intoxication enhances [3H]CCPA binding and does not reduce A1 adenosine receptor function in rat cerebellum. Pharmacol Biochem Behav. 1996;53:249–255. doi: 10.1016/0091-3057(95)00208-1. [DOI] [PubMed] [Google Scholar]
- Crawford CR, Patel DH, Naeve C, Belt JA. Cloning of the human equilibrative, nitrobenzylmercaptopurine riboside (NBMPR)-insensitive nucleoside transporter ei by functional expression in a transport-deficient cell line. J Biol Chem. 1998;273:5288–5293. doi: 10.1074/jbc.273.9.5288. [DOI] [PubMed] [Google Scholar]
- Cully DF, Vassilatis DK, Liu KK, Paress PS, Van der Ploeg LH, Schaeffer JM, Arena JP. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature. 1994;371:707–711. doi: 10.1038/371707a0. [DOI] [PubMed] [Google Scholar]
- Dar MS. Central adenosinergic system involvement in ethanol-induced motor incoordination in mice. J Pharmacol Exp Ther. 1990;255:1202–1209. [PubMed] [Google Scholar]
- Dar MS. Mouse cerebellar adenosinergic modulation of ethanol-induced motor incoordination: possible involvement of cAMP. Brain Res. 1997;749:263–274. doi: 10.1016/s0006-8993(96)01263-2. [DOI] [PubMed] [Google Scholar]
- Dar MS. Involvement of kappa-opioids in the mouse cerebellar adenosinergic modulation of ethanol-induced motor incoordination. Alcohol Clin Exp Res. 1998;22:444–454. [PubMed] [Google Scholar]
- Dar MS. Modulation of ethanol-induced motor incoordination by mouse striatal A(1) adenosinergic receptor. Brain Res Bull. 2001;55:513–520. doi: 10.1016/s0361-9230(01)00552-4. [DOI] [PubMed] [Google Scholar]
- Dar MS. Co-modulation of acute ethanol-induced motor impairment by mouse cerebellar adenosinergic A1 and GABA(A) receptor systems. Brain Res Bull. 2006;71:287–295. doi: 10.1016/j.brainresbull.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Dar MS, Mustafa SJ. Acute ethanol/cannabinoid-induced ataxia and its antagonism by oral/systemic/intracerebellar A1 adenosine receptor antisense in mice. Brain Res. 2002;957:53–60. doi: 10.1016/s0006-8993(02)03599-0. [DOI] [PubMed] [Google Scholar]
- Dar MS, Mustafa SJ, Wooles WR. Possible role of adenosine in the CNS effects of ethanol. Life Sci. 1983;33:1363–1374. doi: 10.1016/0024-3205(83)90819-6. [DOI] [PubMed] [Google Scholar]
- Davies DL, Kochegarov AA, Kuo ST, Kulkarni AA, Woodward JJ, King BF, Alkana RL. Ethanol differentially affects ATP-gated P2X(3) and P2X(4) receptor subtypes expressed in Xenopus oocytes. Neuropharmacology. 2005;49:243–253. doi: 10.1016/j.neuropharm.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Davies DL, Machu TK, Guo Y, Alkana RL. Ethanol sensitivity in ATP-gated P2X receptors is subunit dependent. Alcohol Clin Exp Res. 2002;26:773–778. [PubMed] [Google Scholar]
- Dawson GR, Wafford KA, Smith A, Marshall GR, Bayley PJ, Schaeffer JM, Meinke PT, McKernan RM. Anticonvulsant and adverse effects of avermectin analogs in mice are mediated through the gamma-aminobutyric acid(A) receptor. J Pharmacol Exp Ther. 2000;295:1051–1060. [PubMed] [Google Scholar]
- Dent JA, Davis MW, Avery L. avr-15 encodes a chloride channel subunit that mediates inhibitory glutamatergic neurotransmission and ivermectin sensitivity in Caenorhabditis elegans. Embo J. 1997;16:5867–5879. doi: 10.1093/emboj/16.19.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I, Gordon AS. The role of adenosine in mediating cellular and molecular responses to ethanol. In: Jansson B, Jörnvall H, Rydberg U, Terenius L, Vallee BL, editors. Toward a molecular basis of alcohol use and abuse. Birkhäuser Verlag; Basel: 1994. pp. 175–183. [Google Scholar]
- Diaz-Cabiale Z, Vivo M, Del Arco A, O'Connor WT, Harte MK, Muller CE, Martinez E, Popoli P, Fuxe K, Ferre S. Metabotropic glutamate mGlu5 receptor-mediated modulation of the ventral striopallidal GABA pathway in rats. Interactions with adenosine A(2A) and dopamine D(2) receptors. Neurosci Lett. 2002;324:154–158. doi: 10.1016/s0304-3940(02)00179-9. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Parmentier M, Bertorelli R, Ongini E, Costentin J, Vaugeois JM. Adenosine A2A receptor antagonists are potential antidepressants: evidence based on pharmacology and A2A receptor knockout mice. Br J Pharmacol. 2001;134:68–77. doi: 10.1038/sj.bjp.0704240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele E, Varnier G, Ansaldo MA, Raiteri M. Nicotine administration stimulates the in vivo N-methyl-D-aspartate receptor/nitric oxide/cyclic GMP pathway in rat hippocampus through glutamate release. Br J Pharmacol. 1998;125:1042–1048. doi: 10.1038/sj.bjp.0702130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Ciruela F, Borycz J, Solinas M, Quarta D, Antoniou K, Quiroz C, Justinova Z, Lluis C, Franco R, Goldberg SR. Adenosine A1-A2A receptor heteromers: new targets for caffeine in the brain. Front Biosci. 2008;13:2391–2399. doi: 10.2741/2852. [DOI] [PubMed] [Google Scholar]
- Ferre S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Ferre S, Karcz-Kubicha M, Hope BT, Popoli P, Burgueno J, Gutierrez MA, Casado V, Fuxe K, Goldberg SR, Lluis C, Franco R, Ciruela F. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc Natl Acad Sci U S A. 2002;99:11940–11945. doi: 10.1073/pnas.172393799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Popoli P, Tinner-Staines B, Fuxe K. Adenosine A1 receptor-dopamine D1 receptor interaction in the rat limbic system: modulation of dopamine D1 receptor antagonist binding sites. Neurosci Lett. 1996;208:109–112. doi: 10.1016/0304-3940(96)12577-5. [DOI] [PubMed] [Google Scholar]
- Ferre S, Schwarcz R, Li XM, Snaprud P, Ogren SO, Fuxe K. Chronic haloperidol treatment leads to an increase in the intramembrane interaction between adenosine A2 and dopamine D2 receptors in the neostriatum. Psychopharmacology (Berl) 1994;116:279–284. doi: 10.1007/BF02245329. [DOI] [PubMed] [Google Scholar]
- Ferre S, von Euler G, Johansson B, Fredholm BB, Fuxe K. Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc Natl Acad Sci U S A. 1991;88:7238–7241. doi: 10.1073/pnas.88.16.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB. Adenosine receptors as drug targets. Exp Cell Res. 2010;316:1284–1288. doi: 10.1016/j.yexcr.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Masino SA, Vaugeois JM. Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu Rev Pharmacol Toxicol. 2005;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- Geary TG. Ivermectin 20 years on: maturation of a wonder drug. Trends Parasitol. 2005;21:530–532. doi: 10.1016/j.pt.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gordon AS, Collier K, Diamond I. Ethanol regulation of adenosine receptor-stimulated cAMP levels in a clonal neural cell line: An in vitro model of cellular tolerance to ethanol. Proc Natl Acad Sci U S A. 1986;83:2105–2108. doi: 10.1073/pnas.83.7.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Griffiths M, Beaumont N, Yao SY, Sundaram M, Boumah CE, Davies A, Kwong FY, Coe I, Cass CE, Young JD, Baldwin SA. Cloning of a human nucleoside transporter implicated in the cellular uptake of adenosine and chemotherapeutic drugs. Nat Med. 1997a;3:89–93. doi: 10.1038/nm0197-89. see comments. [DOI] [PubMed] [Google Scholar]
- Griffiths M, Yao SY, Abidi F, Phillips SE, Cass CE, Young JD, Baldwin SA. Molecular cloning and characterization of a nitrobenzylthioinosine-insensitive (ei) equilibrative nucleoside transporter from human placenta. Biochem J. 1997b;328:739–743. doi: 10.1042/bj3280739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack SP, Christie MJ. Adaptations in adenosine signaling in drug dependence: therapeutic implications. Crit Rev Neurobiol. 2003;15:235–274. doi: 10.1615/critrevneurobiol.v15.i34.30. [DOI] [PubMed] [Google Scholar]
- Harvey J, Lacey MG. A postsynaptic interaction between dopamine D1 and NMDA receptors promotes presynaptic inhibition in the rat nucleus accumbens via adenosine release. J Neurosci. 1997;17:5271–5280. doi: 10.1523/JNEUROSCI.17-14-05271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine C, Wegner A, Grosche J, Allgaier C, Illes P, Franke H. P2 receptor expression in the dopaminergic system of the rat brain during development. Neuroscience. 2007;149:165–181. doi: 10.1016/j.neuroscience.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology. 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, Besheer J, Schroeder JP. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology (Berl) 2006;183:429–438. doi: 10.1007/s00213-005-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, Mikusa JP, Hernandez G, Zhong C, Gauvin DM, Chandran P, Harris R, Medrano AP, Carroll W, Marsh K, Sullivan JP, Faltynek CR, Jarvis MF. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther. 2006;319:1376–1385. doi: 10.1124/jpet.106.111559. [DOI] [PubMed] [Google Scholar]
- Hyde RJ, Cass CE, Young JD, Baldwin SA. The ENT family of eukaryote nucleoside and nucleobase transporters: recent advances in the investigation of structure/function relationships and the identification of novel isoforms. Mol Membr Biol. 2001;18:53–63. [PubMed] [Google Scholar]
- Inoue K. Purinergic systems in microglia. Cell Mol Life Sci. 2008;65:3074–3080. doi: 10.1007/s00018-008-8210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Becker HC. Single and repeated episodes of ethanol withdrawal increase adenosine A1, but not A2A, receptor density in mouse brain. Brain Res. 1998;786:80–88. doi: 10.1016/s0006-8993(97)01413-3. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, Subieta A, Van Biesen T, Cartmell J, Bianchi B, Niforatos W, Kage K, Yu H, Mikusa J, Wismer CT, Zhu CZ, Chu K, Lee CH, Stewart AO, Polakowski J, Cox BF, Kowaluk E, Williams M, Sullivan J, Faltynek C. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci U S A. 2002;99:17179–17184. doi: 10.1073/pnas.252537299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinkova I, Vavra V, Jindrichova M, Obsil T, Zemkova HW, Zemkova H, Stojilkovic SS. Identification of P2X(4) receptor transmembrane residues contributing to channel gating and interaction with ivermectin. Pflugers Arch. 2008;456:939–950. doi: 10.1007/s00424-008-0450-4. [DOI] [PubMed] [Google Scholar]
- Jelinkova I, Yan Z, Liang Z, Moonat S, Teisinger J, Stojilkovic SS, Zemkova H. Identification of P2X4 receptor-specific residues contributing to the ivermectin effects on channel deactivation. Biochem Biophys Res Commun. 2006;349:619–625. doi: 10.1016/j.bbrc.2006.08.084. [DOI] [PubMed] [Google Scholar]
- Jennings LI, Hao C, Cabrita MA, Vickers MF, Baldwin SA, Young JD, Cass CE. Distinct regional distribution of human equilibrative nucleoside transporter proteins 1 and 2 (hENT1 and hENT2) in the central nervous system. Neuropharmacology. 2001;10:722–731. doi: 10.1016/s0028-3908(00)00207-0. [DOI] [PubMed] [Google Scholar]
- Jiang LH, Kim M, Spelta V, Bo X, Surprenant A, North RA. Subunit arrangement in P2X receptors. J Neurosci. 2003;23:8903–8910. doi: 10.1523/JNEUROSCI.23-26-08903.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo A, Orlando LR, Grandy DK, Chen JF, Young AB, Schwarzschild MA. Interactions between metabotropic glutamate 5 and adenosine A2A receptors in normal and parkinsonian mice. J Neurosci. 2005;25:10414–10419. doi: 10.1523/JNEUROSCI.3660-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinchuk AV, McCarley RW, Stenberg D, Porkka-Heiskanen T, Basheer R. The role of cholinergic basal forebrain neurons in adenosine-mediated homeostatic control of sleep: lessons from 192 IgG-saporin lesions. Neuroscience. 2008;157:238–253. doi: 10.1016/j.neuroscience.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kaplan GB, Bharmal NH, Leite-Morris KA, Adams WR. Role of adenosine A1 and A2A receptors in the alcohol withdrawal syndrome. Alcohol. 1999;19:157–162. doi: 10.1016/s0741-8329(99)00033-6. [DOI] [PubMed] [Google Scholar]
- Kaur S, Junek A, Black MA, Semba K. Effects of ibotenate and 192IgG-saporin lesions of the nucleus basalis magnocellularis/substantia innominata on spontaneous sleep and wake states and on recovery sleep after sleep deprivation in rats. J Neurosci. 2008;28:491–504. doi: 10.1523/JNEUROSCI.1585-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PP. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA. Allosteric control of gating and kinetics at P2X(4) receptor channels. J Neurosci. 1999;19:7289–7299. doi: 10.1523/JNEUROSCI.19-17-07289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause RM, Buisson B, Bertrand S, Corringer PJ, Galzi JL, Changeux JP, Bertrand D. Ivermectin: a positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor. Mol Pharmacol. 1998;53:283–294. doi: 10.1124/mol.53.2.283. [DOI] [PubMed] [Google Scholar]
- Krauss SW, Ghirnikar RB, Diamond I, Gordon AS. Inhibition of adenosine uptake by ethanol is specific for one class of nucleoside transporters. Molecular Pharmacology. 1993;44:1021–1026. [PubMed] [Google Scholar]
- Labrousse VF, Costes L, Aubert A, Darnaudery M, Ferreira G, Amedee T, Laye S. Impaired interleukin-1beta and c-Fos expression in the hippocampus is associated with a spatial memory deficit in P2X(7) receptor-deficient mice. PLoS One. 2009;4:e6006. doi: 10.1371/journal.pone.0006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Wichert SP, Rossner MJ, North RA, Kirchhoff F, Verkhratsky A. P2X1 and P2X5 subunits form the functional P2X receptor in mouse cortical astrocytes. J Neurosci. 2008;28:5473–5480. doi: 10.1523/JNEUROSCI.1149-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le KT, Babinski K, Seguela P. Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor. J Neurosci. 1998;18:7152–7159. doi: 10.1523/JNEUROSCI.18-18-07152.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated curents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Mailliard WS, Diamond I. Recent advances in the neurobiology of alcoholism: the role of adenosine. Pharmacol Ther. 2004;101:39–46. doi: 10.1016/j.pharmthera.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Manzoni OJ, Manabe T, Nicoll RA. Release of adenosine by activation of NMDA receptors in the hippocampus. Science. 1994;265:2098–2101. doi: 10.1126/science.7916485. [DOI] [PubMed] [Google Scholar]
- Marcoli M, Maura G, Cervetto C, Giacomini C, Oliveri D, Candiani S, Pestarino M. Nitric oxide-evoked cGMP production in Purkinje cells in rat cerebellum: an immunocytochemical and pharmacological study. Neurochem Int. 2006;49:683–690. doi: 10.1016/j.neuint.2006.06.009. [DOI] [PubMed] [Google Scholar]
- McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8:302–330. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Mendelson WB. The sleep-inducing effect of ethanol microinjection into the medial preoptic area is blocked by flumazenil. Brain Res. 2001;892:118–121. doi: 10.1016/s0006-8993(00)03243-1. [DOI] [PubMed] [Google Scholar]
- Meng ZH, Anwer J, Dar MS. The striatal adenosinergic modulation of ethanol-induced motor incoordination in rats: possible role of chloride flux. Brain Res. 1997;776:235–245. doi: 10.1016/s0006-8993(97)00935-9. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodriguez E, Blanco-Centurion C, Gerashchenko D, Salin-Pascual RJ, Shiromani PJ. The diurnal rhythm of adenosine levels in the basal forebrain of young and old rats. Neuroscience. 2004;123:361–370. doi: 10.1016/j.neuroscience.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Naassila M, Ledent C, Daoust M. Low ethanol sensitivity and increased ethanol consumption in mice lacking adenosine A2A receptors. J Neurosci. 2002;22:10487–10493. doi: 10.1523/JNEUROSCI.22-23-10487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy LE, Diamond I, Casso DJ, Franklin C, Gordon AS. Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. J. Biol. Chem. 1990;265:1946–1951. [PubMed] [Google Scholar]
- Nagy LE, Diamond I, Collier K, Lopez L, Ullman B, Gordon AS. Adenosine is required for ethanol-induced heterologous desensitization. Mol Pharmacol. 1989;36:744–748. [PubMed] [Google Scholar]
- Nam HW, Lee MR, Hinton DJ, Choi DS. Reduced effect of NMDA glutamate receptor antagonist on ethanol-induced ataxia and striatal glutamate levels in mice lacking ENT1. Neurosci Lett. 2010;479:277–281. doi: 10.1016/j.neulet.2010.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Newton PM, Messing RO. Intracellular signaling pathways that regulate behavioral responses to ethanol. Pharmacol Ther. 2006;109:227–237. doi: 10.1016/j.pharmthera.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Nicke A, Kerschensteiner D, Soto F. Biochemical and functional evidence for heteromeric assembly of P2X1 and P2X4 subunits. J Neurochem. 2005;92:925–933. doi: 10.1111/j.1471-4159.2004.02939.x. [DOI] [PubMed] [Google Scholar]
- Nishi A, Liu F, Matsuyama S, Hamada M, Higashi H, Nairn AC, Greengard P. Metabotropic mGlu5 receptors regulate adenosine A2A receptor signaling. Proc Natl Acad Sci U S A. 2003;100:1322–1327. doi: 10.1073/pnas.0237126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Offenhauser N, Castelletti D, Mapelli L, Soppo BE, Regondi MC, Rossi P, D'Angelo E, Frassoni C, Amadeo A, Tocchetti A, Pozzi B, Disanza A, Guarnieri D, Betsholtz C, Scita G, Heberlein U, Di Fiore PP. Increased ethanol resistance and consumption in Eps8 knockout mice correlates with altered actin dynamics. Cell. 2006;127:213–226. doi: 10.1016/j.cell.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Olive MF, McGeehan AJ, Kinder JR, McMahon T, Hodge CW, Janak PH, Messing RO. The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C epsilon-dependent mechanism. Mol Pharmacol. 2005;67:349–355. doi: 10.1124/mol.104.003319. [DOI] [PubMed] [Google Scholar]
- Omura S. Ivermectin: 25 years and still going strong. Int J Antimicrob Agents. 2008;31:91–98. doi: 10.1016/j.ijantimicag.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H, Xu T. Partial deletion of the cAMP response element-binding protein gene promotes alcohol-drinking behaviors. J Neurosci. 2004;24:5022–5030. doi: 10.1523/JNEUROSCI.5557-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson FE, Xiong W, Zamzow CR, Chestley T, Mizuno T, Duckworth ML. Transgenic expression of human equilibrative nucleoside transporter 1 in mouse neurons. J Neurochem. 2009;109:562–572. doi: 10.1111/j.1471-4159.2009.05991.x. [DOI] [PubMed] [Google Scholar]
- Phan TA, Gray AM, Nyce JW. Intrastriatal adenosine A1 receptor antisense oligodeoxynucleotide blocks ethanol-induced motor incoordination. Eur J Pharmacol. 1997;323:R5–R7. doi: 10.1016/s0014-2999(97)00147-7. [DOI] [PubMed] [Google Scholar]
- Popova M, Asatryan L, Ostrovskaya O, Wyatt LR, Li K, Alkana RL, Davies DL. A point mutation in the ectodomain-transmembrane 2 interface eliminates the inhibitory effects of ethanol in P2X4 receptors. J Neurochem. 2010;112:307–317. doi: 10.1111/j.1471-4159.2009.06460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portas CM, Thakkar M, Rainnie DG, Greene RW, McCarley RW. Role of adenosine in behavioral state modulation: a microdialysis study in the freely moving cat. Neuroscience. 1997;79:225–235. doi: 10.1016/s0306-4522(96)00640-9. [DOI] [PubMed] [Google Scholar]
- Prediger RD, da Silva GE, Batista LC, Bittencourt AL, Takahashi RN. Activation of Adenosine A(1) Receptors Reduces Anxiety-Like Behavior During Acute Ethanol Withdrawal (Hangover) in Mice. Neuropsychopharmacology. 2006;31:2210–2220. doi: 10.1038/sj.npp.1301001. [DOI] [PubMed] [Google Scholar]
- Proctor WR, Dunwiddie TV. Behavioral sensitivity to purinergic drugs parallels ethanol sensitivity in selectively bred mice. Science. 1984;224:519–521. doi: 10.1126/science.6324348. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Freeman PA, Greengard P, Fienberg AA. Motivational effects of ethanol in DARPP-32 knock-out mice. J Neurosci. 2001;21:340–348. doi: 10.1523/JNEUROSCI.21-01-00340.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta D, Borycz J, Solinas M, Patkar K, Hockemeyer J, Ciruela F, Lluis C, Franco R, Woods AS, Goldberg SR, Ferre S. Adenosine receptor-mediated modulation of dopamine release in the nucleus accumbens depends on glutamate neurotransmission and N-methyl-D-aspartate receptor stimulation. J Neurochem. 2004a;91:873–880. doi: 10.1111/j.1471-4159.2004.02761.x. [DOI] [PubMed] [Google Scholar]
- Quarta D, Ferre S, Solinas M, You ZB, Hockemeyer J, Popoli P, Goldberg SR. Opposite modulatory roles for adenosine A1 and A2A receptors on glutamate and dopamine release in the shell of the nucleus accumbens. Effects of chronic caffeine exposure. J Neurochem. 2004b;88:1151–1158. doi: 10.1046/j.1471-4159.2003.02245.x. [DOI] [PubMed] [Google Scholar]
- Radulovacki M, Virus RM, Djuricic-Nedelson M, Green RD. Adenosine analogs and sleep in rats. J Pharmacol Exp Ther. 1984;228:268–274. [PubMed] [Google Scholar]
- Richard-Lenoble D, Chandenier J, Gaxotte P. Ivermectin and filariasis. Fundam Clin Pharmacol. 2003;17:199–203. doi: 10.1046/j.1472-8206.2003.00170.x. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Roth T. Sleep, sleepiness, and alcohol use. Alcohol Res Health. 2001;25:101–109. [PMC free article] [PubMed] [Google Scholar]
- Sattelle DB, Buckingham SD, Akamatsu M, Matsuda K, Pienaar IS, Jones AK, Sattelle BM, Almond A, Blundell CD. Comparative pharmacology and computational modelling yield insights into allosteric modulation of human alpha7 nicotinic acetylcholine receptors. Biochem Pharmacol. 2009;78:836–843. doi: 10.1016/j.bcp.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Liu XJ. Adenosine in the spinal cord and periphery: release and regulation of pain. Prog Neurobiol. 2003;69:313–340. doi: 10.1016/s0301-0082(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Shan Q, Haddrill JL, Lynch JW. Ivermectin, an unconventional agonist of the glycine receptor chloride channel. J Biol Chem. 2001;276:12556–12564. doi: 10.1074/jbc.M011264200. [DOI] [PubMed] [Google Scholar]
- Short JL, Ledent C, Borrelli E, Drago J, Lawrence AJ. Genetic interdependence of adenosine and dopamine receptors: evidence from receptor knockout mice. Neuroscience. 2006a;139:661–670. doi: 10.1016/j.neuroscience.2005.12.052. [DOI] [PubMed] [Google Scholar]
- Short JL, Drago J, Lawrence AJ. Comparison of ethanol preference and neurochemical measures of mesolimbic dopamine and adenosine systems across different strains of mice. Alcohol Clin Exp Res. 2006b;30:606–20. doi: 10.1111/j.1530-0277.2006.00071.x. [DOI] [PubMed] [Google Scholar]
- Silberberg SD, Li M, Swartz KJ. Ivermectin Interaction with transmembrane helices reveals widespread rearrangements during opening of P2X receptor channels. Neuron. 2007;54:263–274. doi: 10.1016/j.neuron.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Sim JA, Chaumont S, Jo J, Ulmann L, Young MT, Cho K, Buell G, North RA, Rassendren F. Altered hippocampal synaptic potentiation in P2X4 knock-out mice. J Neurosci. 2006;26:9006–9009. doi: 10.1523/JNEUROSCI.2370-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F, Garcia-Guzman M, Karschin C, Stuhmer W. Cloning and tissue distribution of a novel P2X receptor from rat brain. Biochem Biophys Res Commun. 1996;223:456–460. doi: 10.1006/bbrc.1996.0915. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Kiefer F. Drugs for relapse prevention of alcoholism: ten years of progress. Trends Pharmacol Sci. 2008;29:109–115. doi: 10.1016/j.tips.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Stojilkovic SS. Purinergic regulation of hypothalamopituitary functions. Trends Endocrinol Metab. 2009;20:460–468. doi: 10.1016/j.tem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol. 2009;71:333–359. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Saba L, Printz M, Flodman P, Hodgkinson C, Goldman D, Koob G, Richardson HN, Kechris K, Bell RL, Hubner N, Heinig M, Pravenec M, Mangion J, Legault L, Dongier M, Conigrave KM, Whitfield JB, Saunders J, Grant B, Hoffman PL. Genetical genomic determinants of alcohol consumption in rats and humans. BMC Biol. 2009;7:70. doi: 10.1186/1741-7007-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar M, Mallick BN. Effect of rapid eye movement sleep deprivation on 5'-nucleotidase activity in the rat brain. Neurosci Lett. 1996;206:177–180. doi: 10.1016/s0304-3940(96)12453-8. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Delgiacco RA, Strecker RE, McCarley RW. Adenosinergic inhibition of basal forebrain wakefulness-active neurons: a simultaneous unit recording and microdialysis study in freely behaving cats. Neuroscience. 2003a;122:1107–1113. doi: 10.1016/j.neuroscience.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Engemann SC, Sharma R, Sahota P. Role of wake-promoting basal forebrain and adenosinergic mechanisms in sleep-promoting effects of ethanol. Alcohol Clin Exp Res. 2010;34:997–1005. doi: 10.1111/j.1530-0277.2010.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Winston S, McCarley RW. A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic basal forebrain. J Neurosci. 2003b;23:4278–4287. doi: 10.1523/JNEUROSCI.23-10-04278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Johnson J, Heilig M. Effect of the adenosine A2a receptor antagonist 3,7-dimethyl-propargylxanthine on anxiety-like and depression-like behavior and alcohol consumption in Wistar Rats. Alcohol Clin Exp Res. 2007;31:1302–1307. doi: 10.1111/j.1530-0277.2007.00425.x. [DOI] [PubMed] [Google Scholar]
- Trang T, Beggs S, Salter MW. Purinoceptors in microglia and neuropathic pain. Pflugers Arch. 2006;452:645–652. doi: 10.1007/s00424-006-0074-5. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, Inoue K. Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain. 2009;5:28. doi: 10.1186/1744-8069-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28:11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voogd J, Glickstein M. The anatomy of the cerebellum. Trends Neurosci. 1998;21:370–375. doi: 10.1016/s0166-2236(98)01318-6. [DOI] [PubMed] [Google Scholar]
- Wang Y, Haughey NJ, Mattson MP, Furukawa K. Dual effects of ATP on rat hippocampal synaptic plasticity. Neuroreport. 2004;15:633–636. doi: 10.1097/00001756-200403220-00012. [DOI] [PubMed] [Google Scholar]
- Wu J, Lee MR, Choi S, Kim T, Choi D-S. ENT1 regulates ethanol-sensitive EAAT2 expression and function in astrocytes. Alcohol Clin Exp Res. 2010;34:1110–1117. doi: 10.1111/j.1530-0277.2010.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Zhou C, Li K, Davies DL, Ye JH. Purinergic type 2 receptors at GABAergic synapses on ventral tegmental area dopamine neurons are targets for ethanol action. J Pharmacol Exp Ther. 2008;327:196–205. doi: 10.1124/jpet.108.139766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Li C, Weight FF. Inhibition by ethanol of rat P2X(4) receptors expressed in Xenopus oocytes. Br J Pharmacol. 2000;130:1394–1398. doi: 10.1038/sj.bjp.0703439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Sokabe T, Matsumoto T, Yoshimura K, Shibata M, Ohura N, Fukuda T, Sato T, Sekine K, Kato S, Isshiki M, Fujita T, Kobayashi M, Kawamura K, Masuda H, Kamiya A, Ando J. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med. 2006;12:133–137. doi: 10.1038/nm1338. [DOI] [PubMed] [Google Scholar]
- Yao L, Arolfo MP, Dohrman DP, Jiang Z, Fan P, Fuchs S, Janak PH, Gordon AS, Diamond I. betagamma Dimers mediate synergy of dopamine D2 and adenosine A2 receptor-stimulated PKA signaling and regulate ethanol consumption. Cell. 2002;109:733–743. doi: 10.1016/s0092-8674(02)00763-8. [DOI] [PubMed] [Google Scholar]
- Yao L, Fan P, Jiang Z, Mailliard WS, Gordon AS, Diamond I. Addicting drugs utilize a synergistic molecular mechanism in common requiring adenosine and Gi-beta gamma dimers. Proc Natl Acad Sci U S A. 2003;100:14379–14384. doi: 10.1073/pnas.2336093100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, McFarland K, Fan P, Jiang Z, Ueda T, Diamond I. Adenosine A2a blockade prevents synergy between mu-opiate and cannabinoid CB1 receptors and eliminates heroin-seeking behavior in addicted rats. Proc Natl Acad Sci U S A. 2006;103:7877–7882. doi: 10.1073/pnas.0602661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao SY, Ng AM, Muzyka WR, Griffiths M, Cass CE, Baldwin SA, Young JD. Molecular cloning and functional characterization of nitrobenzylthioinosine (NBMPR)-sensitive (es) and NBMPR-insensitive (ei) equilibrative nucleoside transporter proteins (rENT1 and rENT2) from rat tissues. J Biol Chem. 1997;72:28423–28430. doi: 10.1074/jbc.272.45.28423. [DOI] [PubMed] [Google Scholar]