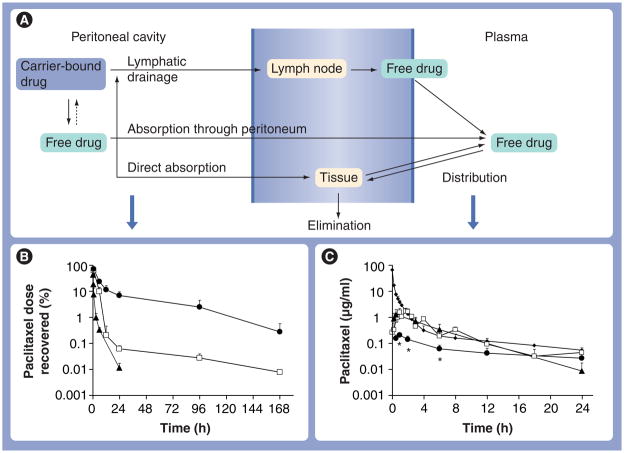
Figure 1. Pharmacokinetic model of disposition of intraperitoneal therapy: effects of the carrier.
Three formulations of paclitaxel, paclitaxel solubilized in Cremophor EL®/ethanol (open squares), paclitaxel-loaded gelatin nanoparticles (triangles) and paclitaxel-loaded polymeric microparticles (circles) were administered by intraperitoneal injections at 10 mg/kg. For comparison, an additional group of mice received an intravenous dose of paclitaxel solubilized in Cremophor EL/ethanol (diamonds). (A) A model of kinetic processes during intraperitoneal treatment. (B & C) Paclitaxel concentration–time profiles in (B) peritoneal lavage samples and (C) plasma samples. Note the different time scales for (B) and (C). At least three mice were used for each time point. Symbols represent means ± standard deviation.
*p < 0.001 compared with other groups by one-way analysis of variance with Tukey post hoc test.
Reproduced with permission from [133].