Abstract
Captopril, an angiotensin-converting enzyme inhibitor used to manage congestive heart failure in the paediatric population, has limited data on efficacy and safety. Its variety of liquid formulations dispensed by different pharmacies poses a challenge in optimizing captopril dosing in patients. Fourteen tertiary paediatric centre pharmacies across Canada were contacted to decipher the type of captopril formulation they dispense, their recipe and the stability of each formulation. Of the 14 centres surveyed, four dispensed solid tablets, two dispensed either solid tablets or liquid formulations, and eight dispensed extemporaneously prepared liquid formulations. There was also great variety in the solutions used to prepare the liquid formulations. The bioequivalence of these preparations has not been studied. As a result, physicians cannot be certain about the effective dose of captopril. Uniformity is recommended among paediatric pharmacies in Canada when preparing medications such as captopril. Proper testing of the stability and bioequivalence of medications is recommended.
Keywords: Captopril, Congestive heart failure, Drug safety, Paediatric
Abstract
On possède peu de données sur l’innocuité et l’efficacité du captopril, un inhibiteur de l’enzyme de conversion de l’angiotensine utilisé pour prendre en charge l’insuffisance cardiaque congestive au sein de la population pédiatrique. Les différentes formulations liquides dispensées dans les diverses pharmacies représentent un problème pour optimiser la dose de captopril chez les patients. Les chercheurs ont pris contact avec 14 pharmacies de centres pédiatriques de soins tertiaires du Canada pour déterminer le type de formulation de captopril qu’elles utilisent, leur recette et la stabilité de chaque formulation. Dans les 14 centres sondés, quatre dispensaient des comprimés solides, deux, des comprimés solides ou une formulation liquide et huit, des formulations liquides « maison ». On constatait également une grande variété dans les solutions utilisées pour préparer les formulations liquides. La bioéquivalence de ces préparations n’a pas fait l’objet d’études. Par conséquent, les médecins ne peuvent pas connaître avec certitude la dose de captopril qui est efficace. Il est recommandé que les pharmacies pédiatriques du Canada visent l’uniformité lorsqu’elles préparent des médicaments comme le captopril ainsi que de procéder à des essais convenables sur la stabilité et la bioéquivalence des médicaments.
Captopril is an angiotensin-converting enzyme inhibitor commonly prescribed for managing heart failure in paediatric patients (1–4). Although believed to be clinically effective in paediatric patients, captopril has not been tested in this population. The data relevant to drug efficacy and safety are based on trials involving adults with acquired heart disease. Hence, the dosing of captopril and its potential toxicity are in question. The only licensed form of captopril is the tablet form; however, many children are unable to swallow tablets (5–8). As a result, there are various formulations prescribed to paediatric patients by different pharmacies. The effect of these formulations on drug absorption, optimal drug dosing and drug toxicity have not been studied. The known side effects of captopril, which may be potentiated by improper dosing, include renal impairment, lowering of blood pressure, oxygen desaturation and chorea (9,10).
At our institution (McMaster Children’s Hospital, Hamilton, Ontario), patients are prescribed captopril tablets. Parents are responsible for dissolving these tablets in water before every dose. This is a recent change from a formulation used in the past. The community pharmacies in the Hamilton region continue to prepare various formulations of captopril. This observation came to light when we were reflecting on interpatient variability in optimizing the captopril dose, along with the inconvenience for patients and their families. We became aware of this fact when we were seeing a five-month old patient discharged from The Hospital for Sick Children (Toronto, Ontario). The patient was originally taking a liquid formulation, but then had to switch to the tablet form (to be crushed and dissolved in water) after transfer of care to our institution. This caused frustration and confusion for the parents, who had been accustomed to one regimen for some time. An article published in the United Kingdom (11) highlighted the potential dangers of using varying liquid formulations that are frequently not licensed. Another survey published in the United Kingdom (12) specifically showed the diversity of captopril formulations used across the country, and its potential effects on drug dosing and toxicity. We decided to perform a survey of the tertiary paediatric centres across Canada to investigate how they dispensed captopril for paediatric patients with heart failure.
METHODS
A total of 14 tertiary paediatric centres across Canada were identified (Table 1). A telephone survey was conducted with a clinical pharmacist responsible for paediatric services, a drug information pharmacist or a compounding pharmacist. The survey aimed to obtain information about the type of captopril formulation they dispensed, their recipe and its stability. The questionnaire is presented below:
Do you dispense tablets for parents to dissolve or a liquid formulation?
If liquid, do you prepare your own formulation?
What is the recipe of your formulation?
How long is your formulation stable for and under what conditions?
TABLE 1.
Tertiary paediatric centres across Canada
| Institution | City | Province |
|---|---|---|
| Janeway Children’s Health and Rehabilitation Centre | St John’s | Newfoundland and Labrador |
| IWK Health Centre | Halifax | Nova Scotia |
| Sainte-Justine UHC | Montreal | Quebec |
| Montreal Children’s Hospital | Montreal | Quebec |
| Children’s Hospital of Eastern Ontario | Ottawa | Ontario |
| Kingston General Hospital | Kingston | Ontario |
| The Hospital for Sick Children | Toronto | Ontario |
| McMaster Children’s Hospital | Hamilton | Ontario |
| Children’s Hospital of Western Ontario | London | Ontario |
| Children’s Hospital of Winnipeg | Winnipeg | Manitoba |
| Royal University Hospital | Saskatoon | Saskatchewan |
| Alberta Children’s Hospital | Calgary | Alberta |
| Stollery Children’s Hospital | Edmonton | Alberta |
| British Columbia Children’s Hospital | Vancouver | British Columbia |
RESULTS
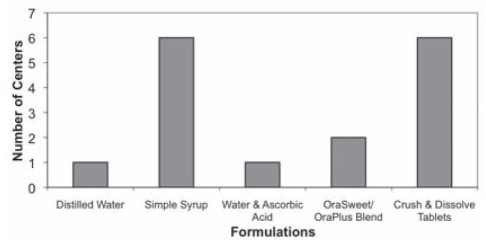
From the survey of 14 centres, significant variability was found in the type of captopril formulations used (Figure 1). Four centres were only dispensing solid tablets to crush and dissolve before each dose. Two centres – the Children’s Hospital of Western Ontario (London, Ontario) and the Stollery Children’s Hospital (Edmonton, Alberta) – provided a choice between solid tablets and a liquid formulation. The remaining eight centres were only dispensing extemporaneously prepared liquid formulations (Table 2).
Figure 1).
Different captopril formulations dispensed by pharmacies across Canada. Ora-Sweet/Ora-Plus Blend, Paddock Laboratories Inc, USA
TABLE 2.
Types of captopril formulations dispensed by the surveyed pharmacies
| City | Institution | Formulation | Solution |
|---|---|---|---|
| Hamilton | McMaster Children’s Hospital | Solid | Water |
| Kingston | Kingston General Hospital | Liquid | Distilled water |
| Ottawa | Children’s Hospital of Eastern Ontario | Solid | Tap water |
| Montreal | Sainte-Justine UHC | Liquid | Simple syrup |
| Montreal | Montreal Children’s Hospital | Liquid | Ora-Sweet/Ora-Plus Blend |
| St John’s | Janeway Children’s Health and Rehabilitation Centre | Liquid | Simple syrup |
| London | Children’s Hospital of Western Ontario | Solid | Water |
| Liquid | Ora-Sweet/Ora-Plus Blend | ||
| Winnipeg | Children’s Hospital of Winnipeg | Liquid | Water and ascorbic acid |
| Calgary | Alberta Children’s Hospital | Liquid | Simple syrup |
| Edmonton | Stollery Children’s Hospital | Solid | Water |
| Liquid | Simple syrup | ||
| Saskatoon | Royal University Hospital | Liquid | Simple syrup |
| Vancouver | British Columbia Children’s Hospital | Liquid | Simple syrup |
| Toronto | The Hospital for Sick Children | Solid | Water |
| Halifax | IWK Health Centre | Solid | Water |
See Table 1 for the province that each hospital is located in. Ora-Sweet/Ora-Plus Blend, Paddock Laboratories Inc, USA
The stability of the various liquid formulations has been studied. Two separate studies (13,14) found that the captopril formulation made with distilled, purified water was more stable than formulations made with other diluents such as sucrose syrup, methylcellulose or sodium ascorbate. Another study by Lye et al (15) found that captopril formulations had greater stability in distilled water compared with tap water, undiluted syrup compared with diluted syrup and formulations containing EDTA compared with those that did not. Overall, a suspension using simple syrup was found to confer the greatest stability (30 days at 5°C) (15). There are, however, no studies measuring the difference in the absorption of captopril with different diluents.
DISCUSSION
Captopril is dispensed in a variety of forms – such as the licensed tablet form to dissolve and unlicensed liquid formulations – in the different tertiary paediatric centre pharmacies across Canada. The liquid formulations have yet to be studied for their bioequivalence. As a result, physicians and patients continue to struggle with dosing adjustments.
When the licensed form of captopril (ie, tablets) is dispensed by pharmacies, it is difficult to ensure that patients are receiving an adequate amount of medication. To completely dissolve the tablet, it must be mixed in water for almost 10 min. It is difficult and inconvenient for parents to administer the medication in this form.
We urge all health care professionals, including paediatricians, subspecialists and pharmacists, to recognize the issues associated with drug formulations and the potential impact on patient safety. We recommend that health care professionals involved in the care of children come together and discuss these issues relating to captopril and other medications, and come to a consensus for an optimal liquid formulation. This formulation should then be studied not only for its stability but also its bioequivalence. If such uniformity were established among the tertiary centres, it would then be transferred to community pharmacies. An excellent example of such an approach is highlighted in the establishment of The Hospital for Sick Children’s formula for preparing a propranolol suspension. Although its absorption and safety have not been tested, the recipe is used uniformly in all of the tertiary centres except the IWK Health Centre (Halifax, Nova Scotia) and Janeway Children’s Health and Rehabilitation Centre (St John’s, Newfoundland and Labrador). Also, the community pharmacies surveyed in Hamilton (Walmart, Dell and West End pharmacies) were all using the same recipe to prepare a propranolol suspension.
The dosing, stability and safety of a medication such as captopril are very important because it is commonly prescribed to paediatric patients. We believe that the present study has highlighted the diversity that exists in the various dispensing forms and demonstrated that steps need to be taken to bring uniformity to formulations so that patient safety and convenience are increased. It may be helpful for a group of paediatric health care professionals across the nation to work together in an effort to solve this important issue of drug formulation.
REFERENCES
- 1.Momma K. ACE inhibitors in pediatric patients with heart failure. Paediatr Drugs. 2006;8:55–69. doi: 10.2165/00148581-200608010-00005. [DOI] [PubMed] [Google Scholar]
- 2.Buchhorn R, Ross RD, Hulpke-Wette M, et al. Effectiveness of low dose captopril versus propranolol therapy in infants with severe congestive failure due to left-to-right shunts. Int J Cardiol. 2000;76:227–33. doi: 10.1016/s0167-5273(00)00384-3. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan S. New drug approaches to the treatment of heart failure in infants and children. Drugs. 1990;39:388–93. doi: 10.2165/00003495-199039030-00005. [DOI] [PubMed] [Google Scholar]
- 4.Sastrosubroto H, Soeroso S, Indrasanto E. Captopril treatment in rheumatic heart disease with congestive heart failure. A preliminary report. Paediatr Indones. 1989;29:209–14. [PubMed] [Google Scholar]
- 5.Michele TM, Knorr B, Vadas EB, Reiss TF. Safety of chewable tablets for children. J Asthma. 2002;39:391–403. doi: 10.1081/jas-120004032. [DOI] [PubMed] [Google Scholar]
- 6.Czyzewski DI, Runyan RD, Lopez MA, Calles NR. Teaching and maintaining pill swallowing in HIV-infected chilren. AIDS Read. 2000;10:88–95. [Google Scholar]
- 7.Meltzer EO, Welch MJ, Ostrom NK. Pill swallowing ability and training in children 6 to 11 years of age. Clin Pediatr (Phila) 2006;45:725–33. doi: 10.1177/0009922806292786. [DOI] [PubMed] [Google Scholar]
- 8.Diamond S, Lavallee DC. Experience with a pill-swallowing enhancement aid. Clin Pediatr (Phila) 2010;49:391–3. doi: 10.1177/0009922809355313. [DOI] [PubMed] [Google Scholar]
- 9.Gantenbein MH, Bauersfeld U, Baenziger O, et al. Side effects of angiotensin converting enzyme inhibitor (captopril) in newborns and young infants. J Perinat Med. 2008;36:448–52. doi: 10.1515/JPM.2008.064. [DOI] [PubMed] [Google Scholar]
- 10.Blackburn ME, Gibbs JL, Dickinson DF. Chorea after cardiopulmonary bypass: Exacerbation by captopril. Int J Cardiol. 1991;30:236–7. doi: 10.1016/0167-5273(91)90104-w. [DOI] [PubMed] [Google Scholar]
- 11.Standing JF, Tuleu C. Paediatric formulations – getting to the heart of the problem. Int J Pharm. 2005;300:56–66. doi: 10.1016/j.ijpharm.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Mulla H, Tofeig M, Bu’Lock F, Samani N, Pandya HC. Variations in captopril formulations used to treat children with heart failure: A survey in the United Kingdom. Arch Dis Child. 2007;92:409–11. doi: 10.1136/adc.2006.109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pramar Y, Das Gupta V, Bethea C. Stability of captopril in some aqueous systems. J Clin Pharm Ther. 1992;17:185–9. doi: 10.1111/j.1365-2710.1992.tb01291.x. [DOI] [PubMed] [Google Scholar]
- 14.Escribano GMJ, Torrado DS, Torrado DJJ. Stability study of an aqueous formulation of captopril at 1 mg/mL. Farm Hosp. 2005;29:30–6. doi: 10.1016/s1130-6343(05)73633-3. [DOI] [PubMed] [Google Scholar]
- 15.Lye MY, Yow KL, Lim LY, Chan SY, Chan E, Ho PC. Effects of ingredients on stability of captopril in extemporaneously prepared oral liquids. Am J Health Syst Pharm. 1997;54:2483–7. doi: 10.1093/ajhp/54.21.2483. [DOI] [PubMed] [Google Scholar]