Fig. 3.
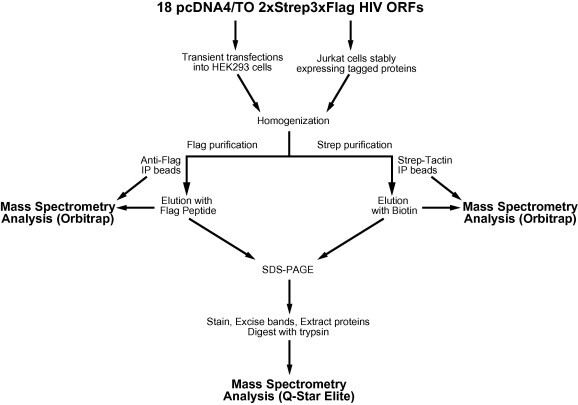
The purification-mass spectrometry strategy to characterize HIV–human protein complexes. Cloned viral genes are inserted into a construct that fuses a 2XStrep3XFlag dual affinity tag on the C-terminus of each factor. These constructs can be used for transient transfection in HEK293 cells or for generating stably expressing Jurkat cells. After lysis, the extract is subjected to either Anti-Flag or Strep-Tactin IP beads where an aliquot of the beads, as well as the elution, is subjected to trypsin digestion and the material is analyzed using the OrbiTrap mass spectrometer. In both cases, a portion of the eluate is also subjected to SDS–PAGE analysis, where the gel is stained, bands excised, proteins extracted and the protein is digested with trypsin and analyzed using a Q-Star Elite mass spectrometer. The data obtained from both sets of purifications and multiple points during the isolation are then integrated together and subjected to an algorithm to derive quantitative viral-host protein–protein interactions. See text for a more detailed description.
